MOJ
eISSN: 2574-819X


Research Article Volume 1 Issue 7
Analytical Chemistry Division, Bhabha Atomic Research Centre, India
Correspondence: Jayshree Ramkumar, Scientific officer, Analytical Chemistry Division, Bhabha Atomic Reasearch Centre, Trombay Mumbai- 400085 India, Tel +9122 2559 2224
Received: November 24, 2017 | Published: December 19, 2017
Citation: Ramkumar J, Chandramouleeswarana S. Metal ion uptake behavior of nafion in presence of organic complexing reagents. MOJ Biorg Org Chem. 2017;1(7):245-247. DOI: 10.15406/mojboc.2017.01.00042
In the present study, uptake studies of transition metal ions like Cu2+ and Co2+ were carried out with Nafion membrane. The effects of different experimental conditions were evaluated. The Nafion membrane was pre-treated by two different ways and used for the studies. The studies were also carried out in presence of complexing agents like ethylenediamine and EDTA. It was seen that the maximum uptake occurred in the pH range of 3-5. The uptake capacity of Nafion was affected by the pre-treatment procedure. This was attributed to the changes of Nafion structure. The presence of different Complexing agents in solution affects the uptake capacity. This could be understood from the strength of the complexes formed by metal ions and thus the reduction in the uptake capacity. The formation of negatively charged complexes by EDTA results in a very low uptake of the metal ions.
Keywords: nafion, transition metal ions, uptake rate, complexing agents
Membrane separation is a very well known technology having a large number of applications. Separation of metal ions by selective membrane transport is of great importance from both fundamental and practical viewpoints. According to IUPAC, a membrane is defined as a heterogeneous phase which acts as a barrier to the flow of molecules and ionic species in liquid or vapour phase. Membranes can be classified into solid and liquid membranes. Ionomers are a class of solid ion exchange membranes with ionic content as high as 10-15 mol%. The most common ionomer is Nafion 117. It is a sulfonated tetrafluoroethylene based fluoropolymer-copolymer possessing excellent water sorption and metal ion transport properties.1 Cellulose nitrate membrane was also used for sorption of metal ions but the solubility of the membrane in acid was used as an advantage in preconcentration techniques for the determination metal ions. A membrane filtration procedure applied for the preconcentration of nickel(II), cadmium(II), copper(II), cobalt(II) and lead(II) ions in which the metal ions were adsorbed on cellulose nitrate membrane filter as their ammonium pyrrolydinedithiocarbamate (APDC) complexes. Then membrane filter was dissolved by using nitric acid for quantification.2 The metal-calmagite complexes on a soluble cellulose nitrate membrane filter were tried for the preconcentration of Cr(III), Co(II), Cu(II), Fe(III) and Pb(II) ions3 The preconcentration/separation procedure is based on chelate formation of the metal ions like Cu(II), Fe(III), Pb(II), Co(II) and Cr(III) with benzopurpurine 4B and on retention of the chelates on cellulose nitrate membrane filter were studied for the determination of these metal ions by dissolving the membranes in acid.4 The heavy metal ions like copper, iron, cobalt, and cadmium were preconcentrated by collected on cellulose nitrate membrane filter as their 8‐hydroxyquinoline complexes and the membrane filter was dissolved by using small amounts of concentrated nitric acid for their determinations.5 The main disadvantage of cellulose nitrate is its solubility in acidic conditions thus making it necessary to look for an alternate. Nafion with its high chemical stability appears as good alternate for metal ion uptake.
The structure of Nafion can be understood by different models. Cluster-network model represents Nafion as consisting of sulphonate ion clusters in the form of inverted micelle with a diameter of 40Å within a continuous fluorocarbon lattice interconnected by narrow channels of 10 Å in width. The other models namely core-shell model describes Nafion consisting of ion-rich core surrounded by an ion poor shell, a rod model where the sulfonic groups arrange into crystal-like rods, and a sandwich model where the polymer forms two layers whose sulfonic groups attract across an aqueous layer where transport occurs. From all the models it is clear that there exists a network of ionic clusters. The degree of hydration is expected to affect the morphology of Nafion. Recent models show that the sulfonic acid functional groups self-organize into arrays of hydrophilic water channels of 2.5 nm diameter dispersed within hydrophobic polymer backbones and allow transport of small ions.
Ion exchange membranes find extensive applications due to their property of perm-selectivity which denotes the difference in permeability between the ions of opposite charges. It is to be mentioned that the perm-selectivity of a membrane is a function of various external parameters. Permeation of neutral substances, heterocyclic bases and cations.6-8 Knowledge of the basic transport phenomena of ions in ion exchange membranes was important for the applications of these membranes. Pretreatment of Nafion membrane prior to its use in study has a strong effect on the ion exchange properties.9-11
In the present study, the uptakes of metal ions (Cu2+ and Co2+) by Nafion under different experimental conditions have been studied. Here the role of permselectivity on the uptake property has been evaluated by studying in presence of various complexing agents. The studies were also carried out using Nafion pretreated differently to see the effect.
Metal ion solutions were prepared by dissolving the appropriate weighed amounts of their chloride salts in dilute acid and standardized by complexometric titrations using suitable metallochromic indicators. The sodium acetate solution was prepared by dissolving 6.8 g of the salt (E. Merck GR) in 500 ml of distilled water to give a stock solution of 0.1 M. This solution was used along with acetic acid for pH adjustments. EDTA and ethylenedaimine solutions (0.1M) were prepared using appropriate amounts of the compounds obtained from BDH AnalaR.
Pretreatment of Nafion membranes were carried out by
The membranes pretreated in the two different ways were labelled N and AT respectively.
The experiments were carried out in batch mode. Weighed pieces (approximately 0.1g) of Nafion membrane, pre-treated differently were equilibrated with 10 mL of 0.01 M of metal ion (Cu2+ and Co2+) solution of known concentration for different periods. The amount taken up evaluated as a function of time.
Nafion membrane has pendant SO3H groups is highly acidic and can be exchanged with other cations. Since Nafion is a cation exchange membrane, pH of the external solution would affect the uptake property of Nafion. From our earlier studies6-8 it was seen that for transition metal ions, the maximum uptake was in the pH range of 3.5-5.5 using sodium acetate buffer medium. Therefore in the present study, the pH was maintained in this range. Normally to achieve selectivity in separation, masking agents are used to complex the interfering ions. Thus it became of interest to see whether the organic complexing agents would affect the uptake property.
In the present study, the uptake of (Cu2+ and Co2+) were carried out in presence of two complexing agents, ethylene diamine(EN) and ethylenediamine tetra acetic acid (EDTA). The results are shown in Figures 1 & 2. The batch mode experiments were carried out with Nafion membranes pretreated differently.
From Figure 1, it is seen that the uptake of copper is affected by the pretreatment of membranes. It is seen that upon pretreatment with boiling acid, the water clusters become bigger and therefore results in lower uptake. This is in correlation with our earlier studies which indicated that the pretreatment resulted in the reduction of ion exchange capacity of the membrane.6,7
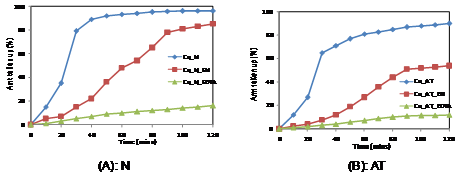
Figure 1 Uptake of copper ions using differently pretreated membranes in presence of different organic complexing agents.
The presence of organic complexing agents in the solution gives very interesting results. The uptake of copper from its pure solution is quite fast and nearly 80% uptake is completed within the first 30 mins. Then it reaches the maximum of 96%. In presence of EN, the rate of uptake is quite low and the maximum amount taken up is 85%. This is due to the fact that ethylenediamine will form a cationic complex with copper ions and therefore the amount of free copper ion in solution for uptake by Nafion is reduced. However with time, the amount taken up increases indicating a possibility of the copper-ethylenediamine complex being taken up. This is possible as the clusters would incorporate the copper ethylenediamine complex. The presence of EDTA in the solution decreases the uptake of copper ion. This is because copper forms an anionic complex and due to properties of permselectivity, Nafion does not take up the negatively charged complex. The small amount of copper in solution could probably be due to the competition of copper between the ion exchange group of Nafion and the EDTA present in solution.
The uptake of Cobalt using the membranes was carried out and the results are given in Figure 2. The uptake of cobalt shows a similar trend to that of copper but the overall amount taken up is lower. In order to understand the changes in the uptake of the metal ions, the rate of uptake was calculated and given in Figure 3. From Figure 3 it was seen that the rate constant values for copper uptake was higher than that of cobalt in all the different cases. This is due to the fact that Nafion has higher uptake capacity for copper as compared to cobalt.
In the present study, effect of complexing agent (EN and EDTA) on the up take of transition metal ions (Cu and Co) were studied. The role of pretreatment of nafion membrane prior to its use was established. The formation of negatively charged complexes by EDTA results in a very low uptake of the metal ions. Ethylene diamine forms a cationic complex which is taken up by nafion. However the kinetics of this sorption is slower than that of pure metal ions. Pretreatment with boiling acids leads to the changes in the clusters present within the nafion resulting in difference in sorption properties.
None.
The author declares no conflict of interest.

©2017 Ramkumar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.