MOJ
eISSN: 2574-819X


Research Article Volume 1 Issue 5
Department of Chemistry, College of Arts, Science and Humanities, India
Correspondence: Harshita Sachdeva, Department of Chemistry, College of Arts, Science and Humanities, Mody University of Science and Technology, Laxmangarh-332311 (Sikar), Rajasthan, India
Received: August 03, 2017 | Published: October 27, 2017
Citation: Sachdeva H, Sharma S. Green preparation and structure elucidation of spiro indole derivatives using grindstone technique. MOJ Biorg Org Chem. 2017;1(5):170-174. DOI: 10.15406/mojboc.2017.01.00031
Grindstone Chemistry technique or Toda’s solvent-free green method of grinding reactants together has been successfully applied for conducting multicomponent reaction of 1H-indole-2,3-dione, malononitrile and 2-thioxo-4-thiazolidinone for the synthesis of Spiro indole derivatives. The reaction conditions have been studied and compared with conventional methods in terms of reaction time, yield and workup procedure. Grinding takes only 15 minutes to complete the reaction. The synthesized compounds have been characterized by IR, 1HNMR, and 13CNMR spectra.
Keywords: grindstone chemistry, solvent-free reaction, spiro indole derivatives, green synthesis, IR, 1HNMR, 13CNMR
One of the most important goals of green chemistry is to redesign environmentally benign synthetic methods which produce less chemical waste. For several years, synthetic chemists have been familiar with the concept of step economy which is the drive to increase the brevity and efficiency of a synthesis by a reduction in the number of synthetic steps. A step economic synthesis has the potential to reduce the number and amount of reagents employed by reducing the number of synthetic steps. Grinding1 is simplified process for conducting the multi-component reactions whereby solvent-free chemical reactions occur by just grinding solid/solid, solid/liquid, or even liquid/liquid reactants together. Required activation energy is provided from friction of the reacting molecules.2 It is not only advantageous from the environmental point of view but also offers rate enhancement, less waste products and higher yields. Such reactions are simple to handle, reduce pollution, and comparatively cheaper to operate.
Grinding3 is interesting because it is performed in the absence of solvent, leading to safe and eco-friendly synthesis. Furthermore, the proposed technique does not require external heating, leading to energy efficient synthesis and is more economical and eco-promising procedure in chemistry. Mechanical-induced breaking of molecular bonds, reduction of particle size, increase in surface area, formation of defects and local melting occur due to the kinetic energy supplied during grinding in solvent-free organic reactions based on grinding. The transfer of very small amounts of energy through friction in this process leads to more efficient mixing and close contact between the starting materials on a molecular scale. The grinding mode for the solid-state reactions has earlier been reported for Reformatsky reaction,4 Aldol condensation, Dieckmann condensation, Knoevenagel condensation,5 reduction, and others.6 Most of these reactions are carried out at room temperature in absolutely solvent-free environment using only a mortar and pestle.7 The unique structural features of Spirooxindoles8 together with varied biological activities have made them honored structures in new drug discovery. Among them, Spiro-pyrrolidinyl oxindoles have been extensively studied as potent inhibitors of p53–MDM2 interaction, finally leading to the identification of MI-888, which could achieve rapid, complete and durable tumor regression in xenograft models of human cancer with oral administration and is in advanced preclinical research for cancer therapy.
Several synthetic Spiroheterocycles containing both indole and pyran rings, possess anticonvulsant, analgesic, herbicidal, and antimicrobial activities.9-12 A number of papers have been published on Spiro indoles incorporating pyran nucleus which include synthesis of
Some of the above mentioned conditions possess shortcomings such as longer reaction time, lower yields, elevated temperature, and formation of side products. These shortcomings led us to develop a safe, environmentally benign, and more efficient method for the synthesis of Spiro indole derivatives. Among nitrogen-containing heterocyclic compounds, thiazoles are of immense interest to medicinal and industrial chemists due to their diverse biological activities19-25 such as antiglutamate, antiparkinson, antimicrobial, anthelmintic, anti-inflammatory, antihyperlipidemic, antihypertension and antioxidant properties as well as inhibition of enzymes such as acetylcholine esterase, aldose reductase, lipoxygenase, ATPase, and HCV helicase. It has been observed that the incorporation of more than one bioactive heterocyclic moiety into a single framework may result into the production of novel heterocycles with enhanced bioactivity. Hence, synthesis of compounds belonging to pyranothiazole series constituent was considered as an important area of research.
In view of the wide range of biological activities associated with indole derivatives and in continuation of our work to develop simple and expeditious procedures for the synthesis of organic compounds,26-30 we wish to report a highly efficient procedure for the synthesis of Spiro indole-pyranothiazole derivatives under solvent-free conditions using grinding technique. Earlier,25 we synthesized these compounds in the presence of piperidine/NiO nanoparticles as catalyst under microwave irradiation. The present work aimed to prepare spiro indole derivatives using green grindstone technique as well as structure elucidation of the prepared compounds using spectroscopic tools. The practical reaction conditions have been studied and compared with our earlier reported method25 in terms of reaction time, percentage yield and workup procedure.
Materials and instruments
Isatin or 1H-indole-2,3-dione (C8H5NO2), malononitrile (C3H2N2) and rhodanine (C3H3NOS2) were purchased from Sigma Aldrich. All melting points were taken on Toshniwal Melting Point Apparatus. 1HNMR and 13CNMR spectra of the prepared spiro indol derivatives were recorded on a Bruker Avance II 400 NMR and Bruker Avance II 100 NMR Spectrometers using deuterated dimethyl sulfoxide (DMSO-D6) and deuterated chloroform CDCl3 as solvent and tetramethylsilane (TMS) as internal reference standard. Also, IR spectra of the prepared compounds were recorded in KBr desk on a Perkin Elmer Infrared L1600300 Spectrum, Two Li-Ta spectrophotometer, 09991423 power cord, manufactured by Perkin Elmer Life and Analytical Science, Shelton, CT, USA in the range of 4000-400 cm-1. Thin Layer Chromatography (TLC) was carried out on silica gel layers. The silica gel plates were activated at 100°C for one hour and stored in a desiccator. Iodine vapors and U. V. chamber were used for visualization of different separated spots on the plate.
Grindstone technique
An equimolar mixture of isatin, malononitrile, and rhodanine (0.01mol) in one drop of piperidine was ground for 10-15 minutes using a mortar and pestle of appropriate size. The initial syrupy reaction mixture solidifies within 15 minutes. It is washed with cold water (to remove impurities) when solid residue was separated out which was filtered, washed with water, dried and crystallized from ethanol to give product. The progress of reaction was monitored by TLC (Benzene: Petroleum Ether, 8:2).
Synthesis of spiro indol derivatives
In this study, we report the development of a solvent-free mechanochemical31,32 approach for the multicomponent33,34 synthesis of a series of Spiro indole derivatives in one step by the reaction of isatin, malononitrile and rhodanine using grindstone chemistry technique as shown in Scheme I. The three starting compounds were mixed in a porcelain mortar at room temperature in the presence of few drops of piperidine and ground for 5-10 minutes. The progress of reaction was monitored by TLC when the reactants were found to have reacted completely in 10 minutes. This syrupy reaction mixture solidifies within 14-15 minutes indicating the completion of reaction to afford Spiro indole pyaranothiazole derivatives in excellent yields. The product could be isolated by just diluting the reaction mixture with ice cold water. This new process is simple, rapid, efficient and inexpensive method for the synthesis of wide range of indole derivatives under solvent-free and mild conditions, making it consistent with the principles of green chemistry. The overall process involves the Knoevenagel condensation35 of 2-thioxo-4-thiazolidinone with 1H-indole-2,3-dione with the elimination of one water molecule (which makes reaction mixture homogeneous) followed by “in situ” Michael addition of malononitrile in a single operation as shown in the mechanistic equation (Scheme II). Michael reaction of malononitrile on exomethylene carbon is interesting as it can afford either a Michael adduct which can exist as such or converted into Spiro pyran system of C-3 of Oxindole (Scheme II).

Scheme I: Synthesis of Spiro indole derivatives using grindstone technique

Scheme II Plausible mechanistic equation for the reaction of 1H-indole-2,3-dione and malononitrile with 2-thioxo-4-thiazolidinone.
Earlier, we reported25 this reaction under microwave irradiation in the presence of 1-2 drops of piperidine as a catalyst. Reaction was completed within 8-10 minutes giving product yield of 70-80 % only. Further, reaction was also carried out under conventional heating which involved refluxing the reactants in absolute ethanol in the presence of 1-2 drops of piperidine for 13-14 hrs giving product yield of 50-60% only. Results are compared with that of our earlier reported method25 and found that grinding method is quite superior to conventional heating or microwave irradiation method as grinding takes only 14-15 minutes with 80-92% yield of the product indicating that such reactions are cheaper to operate and are more economical in organic synthesis. Encouraged by these results, we have extended this reaction to variously substituted 1H-indole-2,3-diones under similar conditions to furnish the respective spiro indole derivatives in excellent yields (80-90 %). Substituted groups, % yield, and melting point of all prepared spiro indole derivatives were summarized in Table 1. It's clear from this table that the maximum yields were obtained with compound 4f which has nito-group (NO2) in position 5 of indole molecule. Again, compound 4f has high melting point of 340 °C. From the data presented in Table 1, it can concluded that both the % yield and melting point of the prepared compounds were affected greatly by the chemical nature of substituted group (R) in isatin or 1H-indole-2,3-dione.
|
Compound |
R |
Time (min) |
Yield (%) |
M.P. (°C)25 |
|
4a |
H |
15 |
90 |
298 |
|
4b |
5-Cl |
15 |
87 |
310 |
|
4c |
5-Br |
15 |
88 |
270 |
|
4d |
5-CH3 |
15 |
88 |
315 |
|
4e |
7-Cl |
15 |
90 |
275 |
|
4f |
5-NO2 |
15 |
92 |
340 |
Table 1 Substituted groups, % yield and Melting point of prepared spiro indole derivatives
Structure elucidation of spiro indol derivatives
IR, 1HNMR, and 13CNMR charts were obtained for the prepared methylated spiro indole derivative (compound 4d) as shown in (Figure 1). IR spectra of this compound displayed characteristic absorption bands in the region 3450-3310 cm-1 (NH str. of NH2), 3265-3115 cm-1 (NH str.), 3059-3008 cm-1 (aromatic C-H str.), 2235-2120 cm-1 (C≡N) and 1720-1685 cm-1 (C=O). Although the reaction proceeds via condensation, there is no broad band appeared in the region 3000-3500 cm-1 indicating that the analyzed compound is water-free and can be described as completely dry compound. This observation may be explained by the evaporation of very small amount of physically adsorbed water molecules during grinding and analysis process. 1HNMR spectrum of 4d showed singlets at δ 10.91 due to NH of indole, multiplet at 6.81-8.02 due to 3H of aromatic ring, singlet at 8.65 due to NH2 of amino group, singlet at 2.84 due to NH of thiazolidinone ring and singlet at 2.11 due to 3H of CH3 group at 5th position of indole ring. Formation of compound 4d was further confirmed on the basis of 13CNMR spectrum. In 13CNMR spectrum, sharp signals were observed at δ 191.54 (C=S), 183.42 (C-NH2), 169.40 (C=O), 144.97-121.00 (aromatic carbon), 117.14 (C≡ N), 59.64 (Spiro carbon) and 22.43 ppm (CH3). All spectral data for compound 4d is summarized in Table 2.
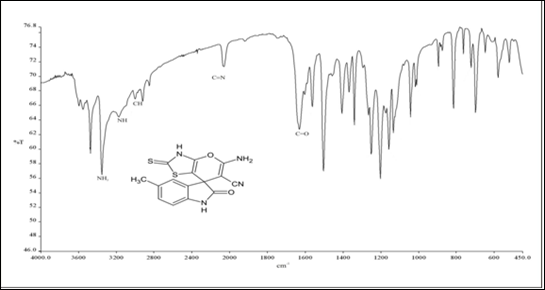

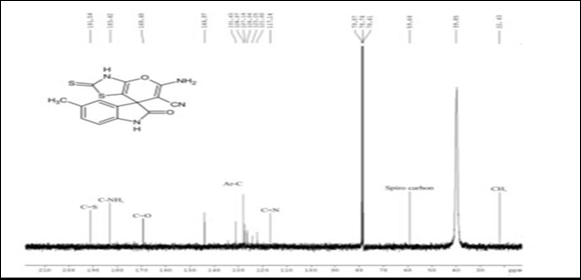
Figure 1 Spectral analysis of Spiro indole derivatives (compound 4d).
Compound 4d: 6′-Amino-2′-thioxo-5-methyl-2-oxo-1′H-spiro {indoline-3, 4′-pyrano [2,3c] thiazole}-5′-carbonitrile.
|
Entry |
IR (cm-1) |
1HNMR (δ ppm) |
13CNMR (δ ppm) |
|
4d |
3350 (NH2), 3125 (NH), 3042-3008 (aromatic C-H str), 2120 (CN), 1689 (CO) |
10.91 (s, 1H, NH indole), 8.65 (s, 2H, NH2), 6.81-8.02 (m, 3H, Ar-H), 2.84 (s, 1H, NH), 2.11 (s, 3H, CH3) |
191.54 (C=S), 183.42 (C-NH2), 169.40 (C=O), 144.97-121.00 (aromatic carbons), 117.14. (C≡N), 59.64 (Spiro carbon), 22.43 (CH3) |
Table 2 Spectral data of Spiro indole derivative (compound 4d)
A simple, faster and efficient protocol for the synthesis of Spiro indole derivatives involving grinding of different Isatins, malononitrile and rhodanine in the presence of piperidine at room temperature under solvent-free conditions is described. This procedure is also consistent with green chemistry approach as no solvent is required (except for recrystallization). Higher yields compared to the conventional method are the advantages of present protocol. Reaction gets completed within 15 minutes in the absence of any solvent in contrast to conventional heating method making it cost effective as it does not require harsh reagents. Reaction can be easily carried out in organic chemistry research laboratory using porcelain mortar.
The authors are thankful to the Dean, Prof. Atul Kumar, CASH, MUST, Laxmangarh (Sikar), Rajasthan, India for providing support and necessary research facilities in the department.
The author declares no conflict of interest.

©2017 Sachdeva, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.