MOJ
eISSN: 2574-819X


Research Article Volume 1 Issue 4
Department of Pharmaceutical and Biochemical Technology, University of São Paulo, Brazil
Correspondence: Mauri Sergio Alves Palma, Department of Pharmaceutical and Biochemical Technology, University of São Paulo, 580, Professor Lineu Prestes Avenue, São Paulo, SP, Brazil, Tel +55–11–3091–2387
Received: July 29, 2017 | Published: September 5, 2017
Citation: Pinheiro DS, Junior ENS, Consolini G, et al. Optimized synthesis and characterization of thiazolidine-2, 4-dione for pharmaceutical application. MOJ Biorg Org Chem. 2017;1(4):122-126. DOI: 10.15406/mojboc.2017.01.00022
Thiazolidine-2,4-dione (TZD) derivatives have great importance for the chemical-pharmaceutical industry, being able to act in diseases such as diabetes mellitus type II, anti-oxidant, anti-inflammatory, anti-HIV and its class of drugs is far from being completed showing an incentive for chemists and pharmacists to study this molecule in order to discover new medicines that can bring benefits to the population. As the commercial TZD presents high purchase value and import difficulty, the TZD synthesis in the laboratory encourages the research, reducing costs and contributing to the process. In this paper, the TZD synthesis process and the comparison of the synthesized TZD with the commercial TZD are shown, in addition to the optimization of the reaction and application of the final product in the synthesis of pharmaceutical intermediates, proving that the synthesized TZD is equivalent to the commercial one being 40 times more economical than commercial TZD.
Keywords: thiazolidine-2,4-dione, organic synthesis, optimization, pharmaceutical intermediates
Thiazolidine-2,4-dione (TZD) was discovered in the 19th century by Libermann et al.1 although its drug class started to be synthesized by 197.2 TZD is a derivative from thiazolidine which belongs to an important heterocyclic group. Besides that, TZD was the first compound that made the thiazol cycle characterization possible, as shown in Figure 1.1
The reaction mechanism proposed by Liberman et al.1 used to obtain thiazolidine-2,4-dione goes through the attack in carbon 2 from chloroacetic acid by the free electrons pair from thiourea sulphur, allowing a 2nd order nucleophilic substitution and subsequent removal of HCl. From that moment a cleavage occurs, by intermolecular nucleophilic substitution, from nitrogen free electron pair over the carboxylic carbon, yielding H2O. At last, the hydrolysis in position 2 of the imino group catalysed by the consequent formation of HCl during the reaction leads to the formation of thiazolidine-2,4-dione (Figure 2).
After structural changes, the TZD derivatives lead to drugs with proven biological activity, as seen in rosiglitazone (commercialized as Avandia®), launched in USA and Europe in 1999, pioglitazone (commercialized as Actos®), launched in USA and Europe in 2000, and also lobeglitazone (commercialized as Duvie®) in the Asian market. Nowadays, lots of TZD condensed with aromatic aldehydes analogues have been synthesized and their respective biological activity linked to their specific structure is elucidated, showing a wide and efficient disease control application.2
The biological activities associated with TZD derivatives are due to the fact that the molecules bond with PPAR gamma (Peroxisome Proliferators Gamma Activated Receptor), a transcription factor that regulates genes associated with lipid metabolism and glycemia regulation. They are also responsible for the inhibition of monocytes and macrophages, the suppression of inflammatory molecules such as interleukins (IL-1 and IL-6), tumor necrosis factor (TNF) and induced nitric oxide synthase (INOS).3
Pharmaceutical and chemical industry uses at most the batch process. In addition, fine chemicals as well as active pharmaceutical ingredients (APIs) have more complex production process and require more stages of reactions in their synthesis, which makes the process more time-consuming and makes it difficult to transpose to industrial scale.4 Considering the importance of the TZD study, this article aims to investigate the possibility of producing the TZD in the laboratory with the same characteristics of the commercial TZD, optimizing its synthesis, and to use the final product as starting reagent of synthesis of pharmaceutical intermediates.
TZD synthesis
The procedure was adapted from Pujar et al.5 A 250 mL three-necked round bottom flask was fixed to a heating blanket. A thermometer was fixed in the bottom round flask, while the other necked was used for insertion of reagents. In the necked of the middle of the flask was coupled a condenser to promote reflux.
Two solutions was prepared: a) In a beaker of 250 mL was solubilized, with stirring, 0.6 mole of chloroacetic acid in 60 mL, being an endothermic solution was observed cooling of the solution; b) 0.6 mole of thiourea was added in 60 mL of water. The chloroacetic acid solution was added, with stirring, to the mixture of thiourea and water. After this procedure, the mixture was stirred for 15 min to ensure greater contact between the phases.
To the mixture was slowly added 60 mL of concentrated hydrochloric acid, with the aid of a separating funnel, to solubilize the solution, which after being solubilized was transferred to the 1 L 3-necked flask attached to the Allhin condenser, the reaction was maintained at reflux for 10 h at a temperature of 110 °C.
After 10 hours of reaction, the flask was cooled to room temperature, crystallized product formed in the form of needles. The flask was kept standing at room temperature to favor crystal formation, and then the flask contents were filtered and washed with water to remove traces of hydrochloric acid and chloroacetic acid. The solid contained in the filter was oven dried at 60 °C and then left in a desiccator. For the purification of TZD, recrystallization was performed with 60 mL of 99.8% ethyl alcohol, and after filtration the pure product was obtained.
(Z)-5-benzilidene-2,4-thiazolidinedione synthesis
The procedure was adapted from Mishra et al.3 A 150 mL round bottom flask was fixed to the heating mantle, with a vertical condenser, thermometer in one side necked and a corked needle in the other side necked. 60 ml of anhydrous ethanol and 0.488 g of TZD (4 mmols) were added to the flask and the reaction medium heated to boiling point of the solvent. Then 405 μL of benzaldehyde (0.424 g, 4 mmol) and 316 μL piperidine (0.272 g, 3.2 mmol) were added and refluxed until the end of the reaction. Then, after the mixture reached room temperature, the reaction medium was cooled in a freezer for crystallization and then recrystallized from the product in ethanol.
(Z)-5-(4-hydroxybenzylidene)-2,4-thiazolidinedione synthesis
The procedure was adapted from Mishra et al.3 A 150 mL round bottom flask was fixed to the heating mantle, with a vertical condenser, thermometer in one side necked and a corked needle in the other side necked. To the flask was added 60 mL of anhydrous ethanol, 0.488 g of TZD (4 mmols) and 0.489 g (4 mmols) of 4-hydroxybenzaldehyde, and the reaction medium was heated to boiling temperature of the solvent. Then 316 μl of piperidine (0.272 g, 3.2 mmol) was added, refluxing until the end of the reaction. Then, after the mixture reached room temperature, the reaction medium was cooled in a freezer for crystallization and then recrystallized from the product in ethanol.
First experiment was performed exactly as described in the literature,5 in a reaction time of 10h, with product yield of 33%. In order to observe the influence of the reaction time and to obtain higher yields of the product, the reaction was carried out in 40h, but presented a product yield of 15%, lower than previously obtained. Therefore, a test was carried out in a shorter reaction time (8h), obtaining product yield of 30%, almost the same yield in 10h of reaction.
In order to optimize the synthesis, a 10h test was performed using a smaller amount of HCl (40 mL) in order to obtain higher quantities of product at the end of the reaction. The product yield obtained was 35%, showing that there is no need to use as much HCl as described in the protocol procedure. Table 1 shows the comparative product yields.
Assays |
Yield (%) |
Assay 1 (10h, 60 mL de HCl) |
33 |
Assay 2 (40h, 60 mL de HCl) |
30 |
Assay 3 (10h, 40 mL de HCl) |
35 |
Table 1 Synthesis assay of TZD
The characterization of the synthesized TZD was first made by measuring the melting point, checking the equivalence with the melting point of the commercial TZD. The comparison of the melting points of the TZDs was performed with a melting point meter model M-560 from Buchi Brazil Ltda. The results obtained are shown in Table 2. The results evidenced the similarity with the values of the literature, allowing them to follow with FT-IR and NMR analyzes.
Analyte |
Melting Temperature Range |
Literature6 |
125-127 °C |
Synthesized TZD |
125.6-127.1 °C |
Commercial TZD |
125.3-127.5 °C |
Table 2 Melting temperature range of TZD
FT-IR analysis was used to identify the chemical bonds of TZD. The values reported in the literature for the FT-IR analysis broadenings are 3132 to 3387 cm-1 for the NH bond, 1681 to 1739 cm-1 for C = O binding and 622 cm-1 for CS binding.7-9 The analyzes were performed on a Fourier transform infrared spectrophotometer (model IR Affinity-1, Shimadzu, Tokyo, JP)The results so obtained can be seen in Table 3 and Figures 3 & 4. The results of the commercial TZD and the one produced in the laboratory were almost the same, and close to the values indicated in the literature, which was yet another indication that the synthesized TZD is equivalent to commercial TZD.
|
Bands FT-IR |
|||
|
N-H |
C=O |
C-S |
|
|
Literature [7-9] |
3132 à 3387 cm-1 |
1681 à 1739 cm-1 |
622 cm-1 |
|
Synthesized TZD |
3469 cm-1 |
1230 cm-1 |
889 cm-1 |
|
Commercial TZD |
3124 cm-1 |
1734; 1649 cm-1 |
889 cm-1 |
Table 3 Bands of FT-IR (KBr disc)
The literature indicates that for analysis of 1H NMR (300 MHz, DMSO-d6) for TZD there are peaks of d 12.02 (s, NH) and d 4.14 (s, CH2).6 For the analysis of 1H NMR for the synthesized TZD, values of d 11.97 (s, NH) for the hydrogen present in the secondary amine of the thiazolidine ring, leaving in the low (disintegrated) field as characteristic of the presence of an electron punch atom like nitrogen and a peak d 4.13 (s, CH2), in the high field (shielded) integrating the two hydrogen of the only methylene present in the molecule (Figure 5). Commercial TZD analysis (Sigma-Aldrich) showed the following results for 1H NMR (300 MHz, DMSO-d6): d 11.98 (s, NH) and d 4.13 (s, CH2) (Figure 6).
As with the melting point and FT-IR results, 1H NMR results showed that TZDs are in accordance with the results reported in the literature. Regarding 13C NMR analyzes for TZD, no results have been found so far in the literature. The 13C NMR results for the synthesized TZD showed peaks at d 173.016 and d 173.786 representing the carbonyls, because they were in the low (disintegrated) field, and the peak d 35.759 peak in the high field (armored), representing the single methylene of the ring (Figure 7).
The analysis of 13C NMR (300 MHz, DMSO-d6) for the commercial TZD demonstrates the equivalence of the TZDs, having values of d 173.0182 and d 173.7860 (C = O) and d 35.7593 (CH2) (Figure 8).
Differential Scaning Calorimetry (DSC) analysis was used to determine the purity of the synthesized TZD. The result showed 99.60% purity, which is observed in Figure 9. The commercial TZD (Sigma-Aldrich) presented a similar result to the TZD mentioned above, with purity of 99.58%, observed in Figure 10. These results provided the necessary confidence to use the TZD synthesized in the laboratory for the next tests, in order to synthesize TZD derivatives under the best process conditions.
In order to verify the economic advantage of synthesizing TZD in the laboratory instead of buying the commercial one, a cost survey was made. The cost of commercial TZD was consulted on Sigma-Aldrich's website (Merck) on 25/07/2017,10 presenting a value of US$ 568.90 for 100g of TZD, resulting in a value of US$ 5.69 / g. The costs of the reagents for the TZD synthesis were quoted with Labsynth, the values and dates of the quotations can be found in Table 4.
Price (US$) |
Quantity |
Quotation Date |
|
Ethyl Alcohol Anhydrous 99.8% P.A. |
113.7 |
20L |
20/12/2016 |
Chloroacetic Acid P.A. |
21.5 |
500g |
22/06/2017 |
Thioureia P.A. |
11.8 |
500g |
22/06/2017 |
Hydrochloric Acid 37% P.A. |
7.5 |
1L |
22/06/2017 |
Table 4 Labsynth quotation of reagents for TZD synthesis
In order to determine the costs for performing a batch reaction, the quantities of reagents described in the Experimental Procedure were considered. Calculations of the cost of each reagent per batch are given below:
The average yield of the reactions performed in the laboratory is 35% after recrystallization, obtaining on average 30 g of TZD purified by batch reaction, therefore, the average cost per gram for TZD synthesized in the laboratory is US $ 0.14/g. The comparison with the price per gram of commercial TZD (US $ 5.69/g) with the average cost per gram of synthesized TZD justifies this synthesis in the laboratory.
Two syntheses of TZD derivatives were made to obtain (Z)-5-benzylidene-2,4-thiazolidinedione and (Z)-5-(4-hydroxybenzylidene)-2,4-thiazolidinedione. Product yields are shown in Figure 11 & 12, respectively.
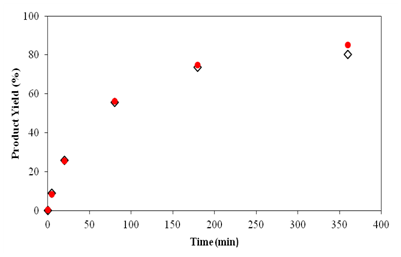
Figure 11 Comparison between the product yields of (Z)-5-benzylidene-2,4-thiazolidinedione obtained by the use of synthesized TZD and commercial TZD with benzaldehyde in 6 hours reaction.
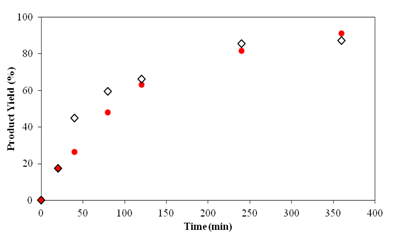
Figure 12 Comparison between the product yields of (Z)-5-(4-hydroxybenzylidene)-2,4-thiazolidinedione obtained by the use of synthesized TZD and commercial TZD with 4-hydroxybenzaldehyde in 6 hours reaction.
Figure 11 & 12 show that the behaviour of the two TZDs are very similar and the product yield at the end of the reaction is very close, 85% and 91% for the synthesized TZD and 80% and 87% for the commercial TZD of the products (Z)-5-benzylidene-2,4-thiazolidinedione and (Z)-5-(4-hydroxybenzylidene)-2,4-thiazolidinedione, respectively. These results confirm the use of TZD synthesized over commercial TZD, especially due to its high cost and difficulty in importing.
With this article, we can conclude that it is feasible to synthesize the TZD in the laboratory with the same quality and specifications of the commercial TZD. In addition, there is a savings of 40 times in the value of the TZD synthesized in relation to the one commercialized. It can also be observed that the tests performed with the synthesized TZD presented results equivalent to the commercial TZD, assuring the use of this TZD for the future tests. Due to the importance of its class of drugs, this article shows an advance in the synthesis of derivatives of TZD, allowing studies of industrial processes.
The authors thank FAPESP, Capes and CNPq for financial support.
The authors declare that there is no conflict of interest regarding the publication of this article.

©2017 Pinheiro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.