MOJ
eISSN: 2574-819X


Research Article Volume 1 Issue 3
Advanced Materials Research Center (CIMAV), Mexico
Correspondence: Norma Flores-Holguin, Advanced Materials Research Center (CIMAV), Miguel de Cervantes 120, CP 31136, Chihuahua, Chih, Mexico, Tel 52–614–4394805
Received: March 27, 2017 | Published: August 22, 2017
Citation: Landeros-Martinez LL, Glossman-Mitnik D, Orrantia-Borunda E, et al. Theoretical calculation of uv-vis, ir spectra and reactivity properties of tamoxifen drug: a methodology comparison. MOJ Biorg Org Chem. 2017;1(3):87-95. DOI: 10.15406/mojboc.2017.01.00017
Some theoretical properties were analyzed and compared with the experimental data for Tamoxifen molecule, a drug commonly used as complementary therapy for breast cancer. The molecular structure and some chemical reactivity parameters were calculated through Density Functional Theory using different functionals, including B3LYP, PBE0, PBEPBE, TPSS, TPSSh and the M05 and M06 density functionals of Minnesota family, all of them combined with a 6-31G (d) basis set. The theoretical IR and ultraviolet-visible (V-Vis) spectra were compared with experimental data. Reactivity parameters are of great importance in the pharmacological testing. For this reason, a correlation between the different chemical models was carried out using the electron affinity and ionization potential calculations. According to the outcomes, the methodology that had a better correlation with the experimental data is M06/6-31G (d).
Keywords: DFT, tamoxifen, vibrational analysis
TAM, tamoxifen; DFT, density functional theory; SERM, selective estrogen receptor modulator; TD-DFT, time-dependent density functional theory; HOMO, highest occupied molecular orbital; LUMO, lowest unoccupied molecular orbital; EA, electron affinity; I, ionization potential; h, chemical hardness; c, electronegativity; w, electrophilicity index; m, chemical potential
Tamoxifen[(Z)-2-(4-(1,2-Diphenyl-1-butenyl)-phenoxy)-N,N-dimethylethanamine] (TAM) has been prescribed to treat patients with breast cancer.1 TAM is a selective estrogen receptor modulator (SERM).2,3 This drug acts as anti-estrogen in breast tissue by interfering with the activity of estrogen: the female sex hormone that promotes the growth of cancer cells in the breast.4–6
On the other hand, it is known that theoretical studies of geometrical, electronic, chemical and spectral properties of drugs are very important as they provide information if the drug has a change in orientation (conformational),7 its reactive behavior,8 the structure-activity relationship9,10 and determine the way the molecule interacts with other species.11
Some authors have studied the structure-activity relationship (QSAR) of TAM,12 which showed that a small difference in the chemical structure can alter the pharmacological activity.13 In recent years, the density functional theory (DFT) calculations reported to provide excellent vibrational frequencies of organic compounds,14 in spectroscopic properties15 and reactivity descriptors of organic and inorganic compounds.16 Also, theoretical studies based on quantum calculations through BLYP/6-31G+(d,p) and B3LYP/6-31G+(d,p) have achieved optimized structures in good agreement with X-ray experimental geometries, and were thus used to investigate the flip-flop process of the TAM propeller and derivatives.17 However, it is important to find a suitable methodology that describes the vibrational properties of TAM, since this will lead to a reliable optimized molecular structure. Besides the detailed knowledge about a structure of drugs, spectra and other properties is necessary for better understanding their chemical and biological.18
The calculation of the vibrational frequencies is a very good aid for the assignment of the IR spectra.19 UV-Vis spectrophotometry is one of the most frequently employed techniques in pharmaceutical analysis. It involves measuring the amount of ultraviolet or visible radiation absorbed by a substance in solution.20 Furthermore, evaluation of UV-Vis may be necessary in the following circumstances: changes in the synthesis of the drug substance; changes in the composition of the finished product and changes in the analytical procedure.20 The wavelength of maximum absorption (lmax) is normally selected.
Moreover, to know the absorption bands of the UV-vis spectrum is very import considering because the quantification of the medicinal substance can be measured by lmax.20 This spectrum also shows the frontier orbital transitions, variations that may lead to the formation of bonds between different atoms. These data are of significant interest for future investigations on the use of carrier vehicles loaded with the drug Tamoxifen or its derivatives.
The main goal of this research was to validate the chemical model that best describes the electronic properties and chemical reactivity parameters of the TAM molecule. Hence, several density functional were selected to calculate the electronic properties, which were compared with experimental results. Thereby, eleven density functionals were evaluated using the 6-31G (d) basis set, reported by Pople et al.21 Some of the defined methodologies were chosen by the experience of previous studies of our research group such as: PBE0 in megazol drug22 and chemical reactivity of pyrrole derivatives;23 B3LYP, M05 and M06 density functionals with Cyanidin molecules24 and the theoretical spectroscopic analysis of Riluzole, using FT-IR, FT-Raman and UV-Vis techniques were obtained with three functionals (B3LYP, MPWLYP and M06-2X) and 6-311++G(d,p) basis set having agreement between experimental and the calculated.25 According of the study reported by Soto-Rojo et al.26 the M06 functional presents the best alternative to obtain the UV-Vis spectra with good accuracy.26 The theoretical determination of chemical model for TAM can be very useful in the design and development of new and better drugs, allowing so that other researchers have the facility to use the chemical model in other derivatives of the drug tamoxifen without validate the chemical model.
All calculations were performed using the Gaussian 09 software.27 The Density Functional Theory (DFT) was used,28–30 which has been proven to be a key method for drug design and specifically in the field of transition metals with medicinal applications, and has been successful in terms of accuracy and reliability.31 The used functionals were: Perdew, Burke and Ernzerhof GGA functional, PBE0;32 GGA hybrid functionals, Becke three parameter Lee, Yang, and Parr, B3LYP,33 and PBEPBE;34 Tao, Perdew, Staroverov, and Scuseria meta-GGA, TPSS;35 Truhlar and coworkers meta-GGA local functional M06-L;26 and hybrid meta-GGA functionals M05-2X,36,37 M05,38,39 M06,36,40 M06-2X,36 M06-HF,41 and TPSSh,35 functionals that have different percentage of Hartree-Fock. These functionals were combined with Pople basis set 6-31G (d)21 for the geometrical optimizations, followed by a frequency calculation to confirm that the structure is at the minimum energy state. The theoretical Infrared spectra (IR) were also plotted with the results obtained by the different chemical model frequency calculations and compared with the experimental IR spectrum.
Ultraviolet-visible spectra (UV-Vis) were calculated by solving the time-dependent density functional theory (TD-DFT) equations42–44 approach and analyzed through the SWizard program;45 theoretical UV-Vis spectra were obtained with different chemical model in the presence of methanol as solvent, using the conductor-like polarizable continuum model (CPCM)46 which defines the cavities as envelopes of spheres centered on atoms or atomic groups. Inside the cavity the dielectric constant is the same as in vacuum, outside it takes the value of the desired solvent;47 the equations were solved for 20 exited states.
The conceptual aspect of the density functional method has been amply used to understand the chemical reactivity, to define a set of chemical concepts such as the electron affinity (EA),48,49 ionization potential (I),48,49 chemical hardness (h),50 electronegativity (c),51,52 electrophilicity index (w)53 and chemical potential (m),53 which are linked to their electronic structure and chemical reactivity. There are different ways to calculate the reactivity parameters. One formulations consists in calculate the ionization potential and electron affinity as the negative of the HOMO and LUMO energy respectively. It is called Koopmans’ theorem. This approximation is widely used with good results.54,55 In the research group of the authors is commonly to calculate with the second approximation, it uses the energy difference between the precursor molecule and the species formed by the adding or removing of an electron. It is the energy difference approximation and considers the energy (E) as a function of the number of electrons (N). The equations of the chemical reactivity calculated by this method are shown in Table 1.
Geometry optimization and Calculated IR spectra
The TAM structure was optimized in gas phase with all density functionals mentioned above, followed by a frequency calculation to confirm that the molecule has a minimum energy conformation, allowing to obtain the theoretical IR spectra, which were compared with experimental results,56 to choose a reliable functional. Figure 1 shows the optimized TAM structure with M06/6-31G (d) methodology.
The optimized structure of TAM shows a non-planar geometry because it has three dihedral angles: C5-C3-C7-C16 with a value of 126.40 degrees, the C5-C4-C3-C8 with -9.32 degrees, and C3-C4-C5-C6 with 171.69 degrees, and a dihedral angle at the opposite end of the molecule, which corresponds to the angle C20-C19-N2-C27 with a value of 69.86 degrees.
The theoretical IR spectra values obtained with the different chemical models were compared with the experimental FT-IR spectrum values. Some of the principal vibrations correspond to different functional groups, such as: Alkane C-H stretching, Alkene -C=C- stretching and C=C ring stretching. The frequency values found for each functional are shown in Table 2. These data were analyzed and the chemical models that reproduce with the best accuracy the experimental data reported by Shivam,56 are M06/6-31G (d) and PBE0/6-31G (d). Figure 2 & 3 show the theoretical Tamoxifen IR spectra for M06/6-31G (d) and PBE0/6-31G (d) respectively.
Vibrations |
|||
|---|---|---|---|
Alkane C-H Stretching cm-1 |
Alkene –C=C- Stretching cm-1 |
C=C ring Stretching |
|
Chemical Model |
Theoretical Data |
||
B3LYP/6-31G(d) |
3029.13 |
1655.32 |
1542.14 |
M05/6-31G(d) |
3008.68 |
1680.05 |
1480.29 |
M05-2X/6-31G(d) |
3014.92 |
1726.33 |
1515.79 |
M06/6-31G(d) |
2929.74 |
1690.54 |
1458.97 |
M06-2X/6-31G(d) |
3061.26 |
1722.71 |
1502.91 |
M06-L/6-31G(d) |
2950.85 |
1655.47 |
1399.23 |
M06-HF/6-31G(d) |
3032.25 |
1747.4 |
1519.65 |
PBE0/6-31G(d) |
2967.06 |
1687.72 |
1496.96 |
PBEPBE/6-31G(d) |
2957.05 |
1596.74 |
1513.66 |
TPSS/6-31G(d) |
3037.74 |
1607.86 |
1515.4 |
TPSSh/6-31G(d) |
3027.43 |
1640.92 |
1539.88 |
Experimental data [48] |
2800-3000 |
1700-1740 |
1400-1500 |
Table 2 Comparison of the theoretical vibrations modes with experimental FT-IR spectrum of Tamoxifen
UV-Visible absorption spectra
The maximum UV-Vis absorption spectra results for Tamoxifen were performed in the presence of solvent, under the same optimization conditions mentioned above. The calculated maximum absorption wavelengths (λmax), the oscillator strength (f), and electronic transitions are shown in Table 3. According to the results obtained in the UV-Vis theoretical spectra the chemicals models that exhibit a better agreement, are M06/6-31G (d) with 27% HF exchange underestimate the λmax by 6 nm, PBE0/6-31G(d) with 25% HF exchange underestimate by 7.8 nm and M05/631G(d) with 25% HF exchange underestimate with 1.8 nm in relation to the 298.2 nm maximum absorption wavelength experimental reported by Merey.54 Figure 4 shows the theoretical Tamoxifen UV-Vis spectra for M06, PBE0 and M05 all 6-31G (d) basis set.
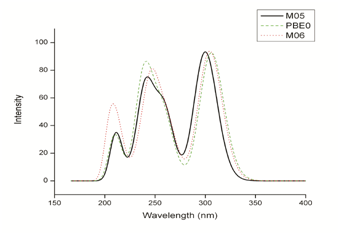
Figure 4 UV-Vis spectra of Tamoxifen calculated with M06/6-31G (d), PBE0/6-31G (d) and M05/6-31G (d).
Chemical Model |
lMax |
F |
Electronic Transitions |
B3LYP |
310.58 |
0.4047 |
H-0->L+0(+98%) |
M05 |
300.01 |
0.4507 |
H-0->L+0(+98%) |
M05-2X |
274.3 |
0.578 |
H-0->L+0(+96%) |
M06 |
304.54 |
0.4536 |
H-0->L+0(+98%) |
M06-2X |
277.63 |
0.56 |
H-0->L+0(+96%) |
M06-L |
358.4 |
0.2268 |
H-0->L+0(+75%) H-1->L+0(21%) |
M06-HF |
246.08 |
0.6775 |
H-0->L+0(+87%) |
PBE0 |
306.07 |
0.448 |
H-0->L+0(+99%) |
PBEPBE |
410.22 |
0.0124 |
H-1->L+0(+74%) H-0->L+0(26%) |
TPSS |
379.75 |
0.0295 |
H-1->L+0(+80%) H-0->L+0(20%) |
TPSSh |
333.76 |
0.3522 |
H-0->L+0(+97%) |
Experimental data [57] |
298.2 |
Table 3 Comparison of the wavelengths (λmax), modes with experimental UV-Vis spectra of Tamoxifen calculated with different chemical model with the 6-31(G) basis set
Variables design and statistical analysis
The chemical models M06/6-31G(d) and PBE/6-31G(d) reproduce adequately the experimental FT-IR spectrum and there is a has good approach to the experimental maximum absorption wavelength with M06/6-31G(d), PBE/6-31G(d) and M05/6-31G(d). Additionally, some reactivity parameters calculation were developed with the eleven functionals mentioned in the computational details section, with the aim to obtain variables that led to develop a statistical analysis of the methodologies and define the best one.
The developed calculated values are electron affinity (EA) and ionization potential (I) and the results are reported in Table 4. In this case all the electron affinity values are negative which means a resonance state or an accepted unbound electron according to Lewars, who states that the electron affinity has negative values when the accepted electron is ejected in microseconds.49
Functional |
EA (eV) |
I (eV) |
B3LYP |
-0.62 |
6.55 |
M05 |
-0.69 |
6.6 |
M05-2X |
-0.73 |
7.03 |
M06 |
-0.56 |
6.67 |
M06-2X |
-0.67 |
7.05 |
M06-L |
-0.4 |
18.8 |
M06-HF |
-0.91 |
7.56 |
PBE0 |
-0.56 |
6.65 |
PBEPBE |
-0.27 |
6.15 |
TPSS |
-0.38 |
6.15 |
TPSSh |
-0.48 |
6.36 |
Table 4 Ionization Potential and Electron Affinity of Tamoxifen with different functionals and 6-31G (d) basis set
Regarding the ionization potential the values are in a range of 6.15 to 7.56 eV with the exception of the value calculated with M06L, with a value of 18.8 eV. This can be attributed to the fact that M06L is the only local functional, with 0% HF exchange, and considering that HF underestimates the ionization potential.58 The only reactivity value found in Tamoxifen is the ionization potential, calculated with B3LYP by Garrido with a value of 6.49 eV.59 In this case, the results obtained with B3LYP, M05, M06 and PBE0 are the closest to the value calculated by Garrido.
A dispersion analysis was done with the results of the reactivity values obtained. It was eliminated the Ionization Potential calculated with M06-L/6-31G(d,) due to the different value compared with the others.
The mean value for the scattering data obtained is -0.59 eV and 6.70 eV for EA and I respectively, while the median has values of -0.59 eV for EA and 6.63 eV for I. These results allowed to calculate the standard deviation for the EA, which exhibits a value of 0.17 and 0.41 for I, and coefficients of variation of 28% and 6.3% respectively. These values represent excellent quality and low variability,60 and contribute to define the best model chemistry. The dispersion analysis results are in Figure 5 where the dash blue line represents the mean value for both, EA and I.
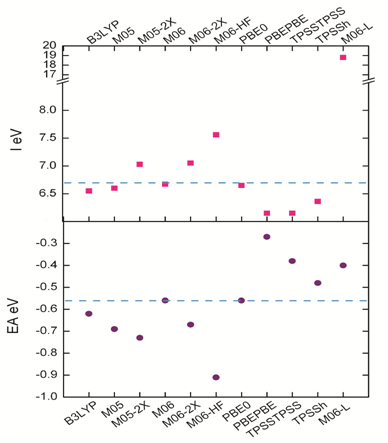
Figure 5 Ionization potential and electron affinity for the model chemistries used in this study and the dispersion between the values.
The dispersion results show that there are two chemistry models with a first-rate approximation for being considered as the best methodology, M06/6-31G (d) and PBE0/6-31G (d). Considering that these two methodologies also have the best agreement with the experimental vibrations and the maximum absorption wavelengths shown above, a third statistical function, the z-score, was used to define the best methodology. This function indicates how many standard deviations an element is from the arithmetic mean.61
According to the results for z-score which are in Table 5, the typical value of the electron affinity scores (zAE) for M06/6-31G (d) and PBE0/6-31G (d) is the same, 0.18. This indicates that both functionals have the same relative position to the mean value. However, typical ionization potential scores (zI) for the chemical models M06/6-31G (d), and PBE0/6-31G (d) are different, being -0.07 and -0.12 respectively. Based on these results M06/6-31G (d) is the best approximation to calculate the Tamoxifen properties. We propose that the chemical model M06/6-31G (d) presents excellent approaches in this analysis.
Functional |
AE (eV) |
I (eV) |
zEA |
zI |
M06 |
-0.56 |
6.67 |
0.18 |
-0.07 |
PBE0 |
-0.56 |
6.65 |
0.18 |
-0.12 |
Table 5 Z score for ionization potential and electron affinity of M06/6-31G (d) and PBE0/6-31G (d) methodologies
Theoretical characterization of Tamoxifen with M06/6-31G (d)
Once the methodology was defined, it was developed, the characterization of the molecule of Tamoxifen that included the structural parameters of minimal energy geometry and a comparison versus experimental bond lengths and angles. Also the electronic properties as electrostatic potential and reactivity parameters.
Structural parameters: We have compared the chemical model M06/6-31G (d) optimized geometry of TAM to the X-ray data62 (Table 6). The analysis the difference between the experimental and theoretical of bond lengths shows that in C-O bond oscillate 0.054 Å, whereas N-C the difference is 0.063 Å and in C-C is 0.063 Å. On the other hand in the bond angles the difference in the C-O-C has 7.53°, in C-N-C 1.29°, while alkene functional group with 0.512°, 0.077° and -0.659° in the bonds angles C3-C5-C9, C3-C7-C15 and C4-C8-C18 respectively. Therefore we have pointed out the good agreement of our theoretical results and the experimental ones.
Electrostatic potential surface: To evaluate the electronic distribution around Tamoxifen, the electrostatic potential surface (EPS) was plotted (Figure 6). The map show the negative potential sites that include electronegative atoms as well as the positive potential sites located around the hydrogen atoms. The main negative center includes the oxygen atom of the ether group and the nitrogen of the amine group. These atoms could be responsible of the interaction through the hydrogen bond with the active site in hormone receptors. This is because this type of bond involves a hydrogen atom that is attracted to a strongly electronegative atom, such as oxygen, fluorine, or nitrogen of a polar covalent bond, in the same or another molecule.63 The contour around TAM is found in the functional groups aryl, ethyl and ether of the drug; these regions most probably constitute the active site of the drug in chemical reactions. The EPS analysts agree with the frontier molecular orbitals study, where highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are located over the phenyl, ethyl and ether groups (Figure 7).
Chemical reactivity: The chemical reactivity parameters obtained for TAM drug are: ionization potential (I), which is defined as the energy needed to remove an electron from a molecule by 6.67 eV; electron affinity (EA),which measures the ability of a molecule to accept electrons or form anions upon -0.56 eV; chemical hardness (h), is the resistance to change its electronic configuration with 3.61 eV; electronegativity (c), represents the tendency of atoms or molecules to attract electrons with 3.05 eV; electrophilicity index (w), that gives an idea of the stabilization in energy when the system acquires electrons from the environment up to saturation by 1.29 eV; and chemical potential (m), measures the tendency of electrons to be released from a system is -3.05 eV. Which they were calculated using the molecular energies of the cationic, ionic and neutral species (Table 7).
Bond Lengths (Å) |
X-Ray |
M06/6-31G(d) |
Bond Angles (°) |
X-Ray |
M06/6-31G(d) |
|||
1O |
12C |
1.4 |
1.357 |
12C |
1O |
20C |
125.7 |
118.165 |
1O |
20C |
1.477 |
1.411 |
19C |
2N |
27C |
114 |
112.031 |
2N |
19C |
1.547 |
1.45 |
19C |
2N |
28C |
112.6 |
110.377 |
2N |
27C |
1.485 |
1.449 |
27C |
2N |
C28 |
109.7 |
109.996 |
2N |
28C |
1.505 |
1.448 |
4C |
3C |
5C |
127.4 |
123.047 |
3C |
4C |
1.5 |
1.355 |
4C |
3C |
7C |
121.8 |
122.493 |
3C |
5C |
1.605 |
1.485 |
5C |
3C |
7C |
108.9 |
114.436 |
3C |
7C |
1.525 |
1.487 |
3C |
4C |
6C |
127.4 |
123.667 |
4C |
6C |
1.569 |
1.508 |
3C |
4C |
8C |
120.3 |
122.081 |
4C |
8C |
1.549 |
1.486 |
6C |
4C |
8C |
112.3 |
114.245 |
5C |
9C |
1.443 |
1.403 |
3C |
5C |
9C |
122.6 |
122.088 |
5C |
10C |
1.457 |
1.396 |
3C |
5C |
10C |
119.7 |
120.368 |
6C |
11C |
1.535 |
1.526 |
9C |
5C |
10C |
117.7 |
117.506 |
7C |
15C |
1.452 |
1.4 |
4C |
6C |
11C |
115.2 |
113.054 |
7C |
16C |
1.453 |
1.399 |
3C |
7C |
15C |
120.8 |
120.023 |
8C |
17C |
1.436 |
1.399 |
3C |
7C |
16C |
121 |
121.75 |
8C |
18C |
1.427 |
1.4 |
15C |
7C |
16C |
118.2 |
118.184 |
9C |
13C |
1.435 |
1.382 |
4C |
8C |
17C |
120.5 |
121.159 |
10C |
14C |
1.406 |
1.391 |
4C |
8C |
18C |
120.1 |
120.602 |
12C |
13C |
1.454 |
1.398 |
17C |
8C |
18C |
119.4 |
118.172 |
12C |
14C |
1.46 |
1.394 |
5C |
9C |
13C |
121.7 |
121.323 |
15C |
21C |
1.398 |
1.388 |
5C |
10C |
14C |
121.9 |
121.947 |
16C |
22C |
1.427 |
1.391 |
1O |
12C |
13C |
115.7 |
115.961 |
17C |
23C |
1.414 |
1.39 |
1O |
12C |
14C |
125.3 |
124.518 |
18C |
24C |
1.414 |
1.389 |
13C |
12C |
14C |
119 |
119.52 |
20C |
19C |
1.529 |
1.514 |
9C |
13C |
12C |
119.5 |
120.248 |
21C |
25C |
1.43 |
1.392 |
10C |
14C |
12C |
120.2 |
119.441 |
22C |
25C |
1.423 |
1.391 |
7C |
15C |
21C |
121.3 |
120.954 |
23C |
26C |
1.419 |
1.391 |
7C |
16C |
22C |
119.9 |
120.967 |
24C |
26C |
1.425 |
1.392 |
23C |
17C |
8C |
120.5 |
120.952 |
8C |
18C |
24C |
120 |
120.956 |
||||
2N |
19C |
20C |
111.1 |
112.469 |
||||
1O |
20C |
19C |
111.6 |
106.643 |
||||
15C |
21C |
25C |
120 |
120.193 |
||||
16C |
22C |
25C |
120.2 |
120.145 |
||||
17C |
23C |
26C |
119.6 |
120.187 |
||||
18C |
24C |
26C |
120.2 |
120.229 |
||||
21C |
25C |
22C |
120.4 |
119.54 |
||||
23C |
26C |
24C |
120.4 |
119.491 |
||||
Table 6 The optimized geometrical parameters (Bond lengths (Å) and angles (°) and experimental of Tamoxifen
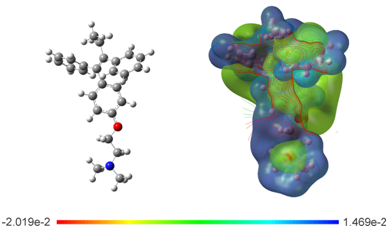
Figure 6 Optimized structure and calculated electrostatic potential map on the molecular surface for TAM drug calculated at the M06/6-31G (d) level of theory. Color range oscillates -2.019e-2 to 1.469e-2: blue, more positive; red more negative.
Drug |
Chemical Reactivity Descriptors |
|||||
I (eV) |
AE (eV) |
c (eV) |
h (eV) |
m(eV) |
w(eV) |
|
TAM |
6.67 |
-0.56 |
3.05 |
3.61 |
-3.05 |
-1.29 |
Table 7 Chemical reactivity parameters with M06/6-31G (d)
Several clinical and experimental studies had been carried out to investigate the complications that occur in postmenopausal states. Ovariectomized Sprague Dawley rats had been used as the model for postmenopausal states in human.33 Duration of the experimental period is worth studying to know the effects of cholesterol diet in short and long term periods. According to the literatures explored, short term period is presumed from 7 days to 4 weeks/less than 5 weeks and long term period is between 5-24 weeks of dietary cholesterol consumption. These durations are widely used in the atherosclerotic related cholesterol diet researches.34,35
The present study used the ovariectomized rats to determine the effect of Noni, with Statin as its positive control on blood pressure, body weight and food intake following eight weeks of consumption of high fat diet containing 2% cholesterol. It was found that there was a significant increase in the body weight by the eighth week when compared to the base line values. No significant difference in the food intake in Ovx control group compared to sham group. The underlying mechanism related to the food intake and body weight changes in ovariectomized group fed with cholesterol is poorly understood. Yet, it was stated that cholesterol levels are strongly linked with the dietary intake. According to the literatures, individuals consuming more cholesterol in daily diet incline to have increased body weight compared to the individuals with normal diet.36 Our finding is in agreement with the previous study which reported that the significant increase in food intake was seen in the experimental rats within the first few weeks of ovariectomy.37 The increase in the body weight following gradual intake of food might most probably be due to the gradual reduction in lean body mass and progressive fat accumulation in different body regions. Progressive fat accumulation induces oxidative stress and was proven to cause obesity, hypertension and cardiovascular diseases.38 Previous study observed that weight gain in the ovariectomized rats was due to the oestrogen deficiency resulting in an increase in the fat accumulation.33 However, the findings are not in line with the present results which showed that Ovx group did not exhibit any significant increase in the body weight compared to the sham group. It can be explained that oestrogen has less influence on the body weight in early onset of postmenopausal state. Based on the present study, the increase in body weight mainly reflects the consumption of cholesterol diet. It is proven that the effect of oestrogen deficiency is further worsened with the additional cholesterol intake. Weight gain among the ovariectomized rats would probably happen with time.
Oestrogen deficiency causes a significant increase in the total cholesterol and triglycerides, which affects lipoprotein metabolism, platelet aggregation and vessel resistance.39 Previous studies observed that the intake of cholesterol-rich food develops obesity and several cardiovascular complications in various animal models.40,41 These high cholesterol diets induced damage to the endothelium of large arteries and heart causing hypertension and subsequently, producing atherosclerosis resulting in coronary heart disease.42
In the present study, it is shown that there is no difference in the systolic, diastolic and mean blood pressure in the Ovx group. It was observed that consumption of additional 2% cholesterol diet for eight weeks caused no significant changes in the blood pressure measurement. The oestrogen deficiency following ovariectomy and increase cholesterol intake are the predisposing factors of the obesity. The incidence of hypertension frequently occurs in obese women with poor diet control, modern life style, existing family history and menopausal age.43 Therefore, it is most likely that ovariectomized rats fed with 2% cholesterol diet would develop hypertension. No significant changes were observed in the Ovx group was most likely due to its early onset of postmenopausal state. Oestrogen deficiency is believed to affect the physiological changes after prolonged onset. However, lack of oestrogen along with consumption of cholesterol diet may worsen the above findings even in the early onset.
In a routine research on cardiovascular disease, ovariectomized rats fed with 2% cholesterol had been used to develop the atherosclerotic animal model. The experiments related to this type of animal models are needed to be carried out for prolonged period (up to 24 weeks)34,35 in order to achieve the significant outcomes of atherosclerosis or hypertension. Based on the present findings, it is proven that the use of 2% cholesterol diet caused no such significant increase in food intake, body weight and blood pressure in ovariectomized rats over eight weeks (short term period) following ovariectomy.
In this work, different chemical models were implemented to determine the structural parameters and reactivity of TAM. The M06/6-31G (d) and PBE0/6-31G (d) chemical models had the best approach to the FT-IR spectrum for maximum absorption wavelengths the best results were obtained with M06/6-31G(d), PBE0/6-31G(d) and M05/6-31G(d). These chemical models yielded better results for modeling the structural parameters studied. The performed statistical analysis of the electron affinity and ionization potential reactivity parameters showed that has the closest approximation to the mean value is for M06/6-31G(d). Therefore, it was defined as the best methodology. The calculation of the structural parameters with this methodology was in good agreement with the experimental values and the electrostatic potential surface determined which moieties of the molecule will interact with the active site of the hormone receptor.
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT) and Centro de Investigación en Materiales Avanzados, S.C. (CIMAV). LLLM gratefully acknowledge a fellowship from CONACYT. EOB is a researcher for CIMAV and CONACYT and NFH is a researcher of CIMAV and CONACYT.
The author declares no conflicts of interest.

©2017 Landeros-Martinez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.