MOJ
eISSN: 2574-9722


Short Communication Volume 1 Issue 2
1Department of Physics, University of Mount Allison, Canada
2Department of Chemistry and Biochemistry, University of Mount Allison, Canada
Correspondence: Khashayar Ghandi, Department of Physics, University of Mount Allison, Canada
Received: May 04, 2017 | Published: May 18, 2017
Citation: Ghandi K, Mahimwalla Z. Plasma induced thin films of linalool are not polylinaool. MOJ Biol Med. 2017;1(2):31–32. DOI: 10.15406/mojbm.2017.01.00008
In a recent paper Mohan V. Jacob et al.1 reported fabrication of thin films made from Linalool (an antioxidant) on glass using ignite glow discharge from a 13.56MHz RF generator with different powers. Our analysis shows that the RF plasma induced reaction of the biologically important natural based molecule, Linalool, leads to very rich and unexpected surface chemistry instead of a simple homopolymer made from linalool. This leads to a much more complex product that while it is not homo linalool polymer it has very interesting physical properties.
Keywords: surface chemistry, RF induced chemistry, linalool.
Free radicals (FR) and reactive oxygen species (ROS) can be involved in many diseases2 while they can also be used to treat cancer.3 One way to neutralize FRs and ROS is via antioxidants.2 Plants create many antioxidants which some are in the form of terpenes and terpenoids which are ubiquitous high vapour pressure chemical compounds that are typically insoluble in water. In recent papers Mohan V. Jacob et al.3 reported fabrication of thin films made from some terpene based essential oil components on glass substrates using ignite glow discharge from a 13.56MHz RF generator.3,4 The authors showed thin film formation with very interesting physical properties. E.g. refractive indices of the thin films were very similar to that of glass. We argue that the thin film formation could be due to impact of very interesting surface effects on RF based chemistry.3
The only chemical characterization Jacob et al.1,3 have was via FTIR spectra. Although the authors did characterize many physical properties of the thin films, they did not have enough chemical structure characterization of their thin film made from Linalool and as such it was not clear what the thin film material is chemically, and if it is indeed a polymer what that polymer was. For the case of linalool authors showed the presence of both C=O group and branched structure with increased methyl groups and decreased amount of methylene groups compared to what exists in Linalool as well as the presence of both types of C=C presented in their spectra.3,4
As such the product cannot be due to free radical propagation chain reaction on linalool (typical polymers made via classical free radical polymerization) since such a product would not have C=O and in the propagation step of polymerization at least there would be addition of the propagating radical to one of the –C=C– which turn it into –CHR-CH2– (which means addition of methylene groups) although in many cases both C=C bonds would turn into –CHR-CH2– and the product would have a large fraction of methylene groups. This means the number of C=C bonds decrease by a factor 2 at least and it could be as little as 0. (Figure 1) shows the poly-3,7-dimethyl-octene-poly-3ol polymer (Linalool Homopolymer) which is expected from bulk free radical polymerization of Linalool, when such polymerization can happen on one double bond.
If the reaction propagates from both double bonds, no double bond would be left in (Figure 1).As such the chemical reaction that is going on the substrate is a combination of surface induced derivatization (which could well be ionic reactions) followed by other types of chemical bonding to other monomers (hence the existence of C=O and the breaking of C-C bonds in4 and presence of C=C peaks reported in the case of3). There could as well be a very large degree of fragmentation reaction with glass surface acting like a catalyst as well as atom transfer reactions and removal of hydrocarbons in favour of enhanced hydrogen bonding in the molecule. This is also consistent with significant increase in OH functional group observed in the IR spectra in Refs3 and.4 One possibility is that the reaction involves H atom abstraction from the methylene group followed by recombination with molecules bonded on the surface. It is very likely that combination of plasma and surface makes the glass surface serve as a catalyst towards dissociation of the molecule and as well formation of O centered radicals (via H atom abstraction) which also can rearrange to form C=O bonds.
Since in Refs3 and4 there is thin film formation on a substrate under RF the authors indeed have a more complex product than a simple homopolymer such as the one in (Figure 1) on the surface and it could even have been a surface reaction that yields glassy ionic products. While the studies of the authors is interesting and has significant potentials for very interesting and rich surface chemistry and turning the biological products into useful optical products, the product formed from reaction of linalool on the surface (based on their reported spectra) is certainly different with what is expected from ordinary Homopolymer expected from free radical polymerization shown in (Figure 1), and it is either an ionic compound with strong intermolecular interactions on the surface or a complex derivative of linalool with C=O and C=C4 and reduced methylene groups.3,4
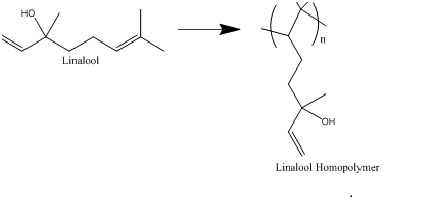
Figure 1 The polymer that should be formed from linalool via free radical polymerization via one double bond.
In conclusion, using ignite glow discharge from a 13.56MHz RF (like what used by Jacob et al.1,3) can lead to tuning very complex surface chemistry to make novel products from biological material; materials that would be very different with homo-polymers and it would not lead to simple homo-polymers such as the one in (Figure 1). Such surface induced plasma studies indeed can lead to new chemistry that is not available otherwise but needs to be investigated thoroughly.
None.
The author declares no conflict of interest.

©2017 Ghandi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.