MOJ
eISSN: 2574-9722


Research Article Volume 10 Issue 1
Department of Anatomy, Faculty of Basic Medical Sciences, College of Medicine and Allied-health Sciences, Bingham University, Karu, Nasarawa State, Nigeria
Correspondence: Emmanuel Enyojo Oguche, Department of Anatomy, Faculty of Basic Medical Sciences, College of Medicine and Allied-health Sciences, Bingham University, Karu, Nasarawa State, Nigeria, Tel +2348039683301
Received: January 23, 2025 | Published: February 10, 2025
Citation: Elijah SO, Oguche EE, Ujah WO, et al. Effect of curcumin on bisphenol-a induced cytotoxicity in the jejunum of adult wistar rats. MOJ Biol Med. 2025;10(1):14-18. DOI: 10.15406/mojbm.2025.10.00234
Bisphenol A (BPA), a ubiquitous environmental contaminant, has been implicated in various adverse health effects, including cytotoxicity in the gastrointestinal tract. This study investigated the effect of curcumin, a natural polyphenol with antioxidant and anti-inflammatory properties, on BPA-induced cytotoxicity in the jejunum. Bisphenol A has been associated with obesity, inflammation, oxidative injury, villous rupture and atrophy. Twenty adult wistar rats were divided into four groups: group one served as the control group which was administered 0.5ml of sunflower oil, group two received only bisphenol A (65mg/kg), group three received bisphenol A (65mg/kg) and curcumin (100mg/kg) and group four was administered only curcumin (100mg/kg) dissolved in 0.5ml of sunflower oil for a period of 28 days. Bisphenol A induced an increase in malondialdehyde and high sensitivity-c-reactive protein concentration and a decrease in glutathione levels. The groups administered curcumin presented a decrease in malondialdehyde and high sensitivity-c-reactive protein and a significant increase in glutathione levels. Histological observation of the jejunum revealed presence of amyloidosis, few goblet cells and shrunken villi in group two. Group three showed absence of amyloidosis, larger and unbroken villi while group four showed absence of amyloidosis with a very fine epithelium and intact villi. These findings showed that bisphenol A had cytotoxic effect on intestinal cytoarchitecture and curcumin holds potential as a protective agent against BPA-induced cytotoxicity in the jejunum, potentially contributing to strategies for mitigating the adverse effects of environmental contaminants on gastrointestinal health.
Keywords: bisphenol A, cytoxicity, curcumin, duodenum, wistar rats
BPA, bisphenol A; NIEHS, national institute of environmental health sciences
Polycarbonate material and epoxy resins are produced vastly with the aid of a monomer called bisphenol A.1 This monomer comes in contact with animals due to its often involvement in the packaging, preservation and storage of food stuff and drinks in plastic containers, plastic bags and even cans.2 According to the National Institute of Environmental Health Sciences (NIEHS), bisphenol A is present in some water bottles, baby bottles, dental fillings and sealants, dental and medical devices, safety equipment, compact discs, household electronic items and sports equipment because of its simple chemical structure which makes it easier to manipulate.3 Bisphenol A is present in manufacturing of items made up of polycarbonate plastic and epoxy resins. It is clear and transparent which is why it is used regularly to line containers and cans. It is also really easy to deform and manipulate, placing it as the most used monomer in the production of plastic. The grounds used to classify bisphenol A as a harmful substance was the fact that hydrolysis of polycarbonates expel bisphenol A at high temperature.4 The bioactivity of bisphenol A has been researched a lot but few of the researchers examined its effect on the small intestines since its common route of contact with organisms is orally. Studies have shown that this is also a major reason to avoid microwaving of foods in plastic containers and using a dish washer to cleanse plastic containers. In mammals, bisphenol A is an endocrine disruptor that participates in several inflammatory and infectious diseases.4 The response to injury, infection or both is known as inflammation, it is also micro-circulation dependent. The micro-vascular components which are arterioles, capillaries and venules undergo changes during inflammation to aid delivery of inflammatory cells which include; activated monocytes, macrophages.5 Bisphenol A is said to increases the expression of pro-inflammatory mediators NO and PGE2 including its other upstream factors iNOS, COX2, cPLA2. bisphenol A induces the phosphorylation and nuclear translocation of NF-kBp65 by degrading the IkB.6
Introduction of foreign substances like bacteria, fungi or viruses, whether orally or through injuries can cause inflammation. Redness, swelling, pain and tenderness are the four cardinal signs of inflammation which occur at the tissue level. Inflammation is mediated by molecules that facilitate inflammatory response5 including vasoactive amines such as histamine and serotonin, peptide such as bradykinin and eicosanoids which include prostaglandins, leukotriene and thromboxane. Inflammation plays a crucial role in the occurrence and development of disease, and compounds such as curcumin which have anti-inflammatory effects are the direction to look, for the production of therapeutic drugs.7 it has been reported that bisphenol A increases the expression of pro-inflammatory mediators NO and PGE2 including its other upstream factors iNOS, COX2, cPLA2. bisphenol A induces the phosphorylation and nuclear translocation of NF-kBp65 by degrading the IkB.6 Bisphenol A is known to threaten the integrity of the intestinal barrier due to its inflammatory effect.8 This does not only affect the intestines ability to regulate permeability of water, ions but also affect the intestinal villi’s ability to sufficiently absorb nutrients. Absorption of nutrients will be affected because these finger like projections (microvilli) which extends into the lumen of the small intestine consists of many enterocytes which form the striated bush or border become inflamed by the inflammatory effect caused by bisphenol A. It is alarming that the individuals that make the most dietary contact with bisphenol A are infants as a result of manufacturers using polycarbonate plastic to produce feeding bottles. Epoxy resins are used as a sealant in food storage cans which are used to store milk and baby formulas.9
Curcumin causes anti-inflammatory effects by modulating inflammatory signaling pathways and inhibiting the production of inflammatory mediators. It regulates the nuclear factor kappa-B,10 mitogen activated protein and other inflammatory pathways resulting to its anti-inflammatory effect. A study carried out exploring the anti-inflammatory properties of curcumin revealed that it decreases the levels of pro-inflammatory mediators such as Interleukin-1, IL-1β, IL-6, IL-8, IL-17, IL-27. A popular relative of inflammation is oxidative stress which is caused by the accumulation of Reactive oxygen species which aids inflammation by activating transcription factors associated with inflammation.11 Curcumin is an anti-oxidant, it reduces inflammation via its anti-oxidant activity by reducing the reactive oxidation species (ROS) by its inhibiting effect on nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and increasing the release of anti-oxidant enzymes.12–14 The bioavailability of curcumin plays a major role on how to enjoy its anti-inflammatory and anti-oxidative benefits.15,16 Curcumin administered in combination with piperine was associated with a noteworthy reduction in the erythrocyte malondialdehyde content and a significant increase in glutathione levels in patients with tropical pancreatitis.17 It also regulates Mitogen-activated protein kinases and other signaling pathways (MAPK).18–20 The nuclear factor Kappa B(NF-ĸB) pathway is a key mediator f inflammation, other factors of inflammation are cytokines, chemokines, adhesion molecules and acute phase proteins.10 Administration of any substance orally must have an effect on the rest of the body system because whatever substance the small intestines allow to be introduced into the blood stream will circulate round the body via the blood vessels, leaving each system to experience its own kind of aberration.8
Ethical approval for Study
All protocols on animal handling strictly followed the guidelines of the Institutional Animal Care and Use Committee (IACUC) as approved by the BHU Ethics Review Committee, Bingham University, Karu, Nasarawa State, Nigeria.
Study material procurements
|
Group |
Treatment |
Feeding |
|
Group I (control) |
Food + distilled water + sunflower oil only |
28 days |
|
Group II (Bisphenol A) |
Distilled water + food + Bisphenol A dissolved in sunflower oil at a dose of 65mg/kg body weight orally |
28 days |
|
Group III (Bisphenol A+ Curcumin) |
Distilled water + food + Bisphenol A dissolved in sunflower oil at a dose of 65mg/kg body weight and Curcumin dissolved in sunflower oil at a dose of 100mg/kg body weight orally |
28 days |
|
Group IV (Curcumin) |
Distilled water + food + Curcumin dissolved in sunflower oil at a dose of 100mg/kg body weight orally |
28 days |
Table 1 Experimental design
Result shows relative increase in body weight across group between the initial body weight of animal and their final body weight but body weights of animals treated with both bisphenol A and curcumin (group three) and curcumin only (group four) gained less weight compared to the other groups. There was a relative increase in body weight of the control group (group one), while the group treated with bisphenol A only (group two) showed high increase in body weight Figure 1,2.
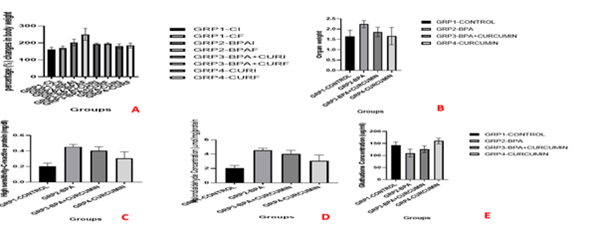
Figure 1 Diagrammatic representation of information concerning weight changes (A), organ weight (B), blood parameters (C) and biochemical parameters (D & E).
A - Changes in body weight of animals
B-Effect on organ weight of animals
D-Effect on Malondialdehyde Concentration.
C-Effect on High Sensitivity-C-Reactive Protein concentration in blood serum.
E-Effect on glutathione Concentration
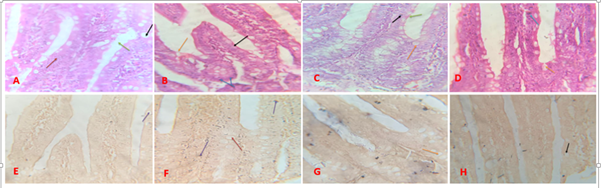
Figure 2 Histology And Special Stain Analysis.
A- Photomicrograph of jejunum of control group showed regular villi with unbroken epithelia lining (brown arrow) and goblet cells (black arrow). Crepts (green arrow). (H & E. X400). B-Photomicrograph of jejunum of rats administered BPA showed shrunken villi (black arrow). Inflammatory cells present (blue arrow), ruptured villi (orange arrow) and few goblet cells (green arrow). (H & E. X400).
C- Photomicrograph of jejunum of rats administered BPA + Curcumin showed normal villi (green arrow). Enterocytes (black arrow) and goblet cells (orange arrow). (H& E. X400).
D-Photomicrograph of jejunum of rats administered curcumin showed normal villi with goblet cells (black arrow) with fine epithelium (blue arrow). Goblet cells (orange arrow) and enterocytes (blue arrow). (H & E. X400).
E- Photomicrograph jejunum of control group showed consistent villi with epithelia lining (blue arrow) and goblet cells (black arrow). No amyloid deposit. (Congo red. X400).
F - Photomicrograph of jejunum of rats administered BPA shows amyloid plaques (purple arrow). Few goblet cells (red arrow). (Congo red. X400).
G - Photomicrograph jejunum of rats administered with BPA + Curcumin shows no amyloid deposits with goblet cells (orange arrow). (Congo red. X400).
H - Photomicrograph of jejunum rats administered with curcumin did not show amyloid deposit for degenerative changes. Goblet cells (black arrow). (Congo red. X400)
The control group showed relative weight gain. The group administered bisphenol A only showed the highest increase in weight. This agrees with the study by Naomi et al.,21 that bisphenol A induces obesity via direct action on peroxisome proliferator-activated receptor gamma (PPAR gamma) and other signaling pathways. The group treated with bisphenol A and curcumin had the least increase in weight. It was deduced that curcumin’s weight managing abilities is as a result of the up-regulatory effect curcumin has on Peroxisome proliferator-activated receptor gamma (PPARγ),22,23 which regulates adipogenesis.21 Hence, curcumin serves as a remedy to the destructive effect bisphenol A has on Peroxisome proliferator-activated receptor gamma (PPARγ),21 and is also a pathway by which curcumin manages weight and prevents unproportioned weight gain. The group administered curcumin only showed lesser proportional weight gain compared to the control group. This means that curcumin has weight managing abilities by increasing energy expenditure and suppressing cortisol levels in adipocytes as reported by Kasprzak-Drozd et al.24
High-Sensitivity-C-reactive protein (HSCRP) is a non-specific marker of inflammation where significantly higher figures indicate intestinal inflammation and injury.25 The high-sensitivity-C-reactive protein concentration was highest in the group treated with bisphenol A only which agrees with the study by Choi Ha et al.,26 that bisphenol A is significantly related to increased high-sensitivity-C-reactive protein concentration. The group administered only curcumin had the least concentration of high-sensitivity-C-reactive protein and agrees with the study by Gorabi et al.,27 that curcumin is beneficial in decreasing high-sensitivity-C-reactive protein concentration. The group treated with bisphenol A and curcumin also had a high concentration of high-sensitivity-C-reactive protein compared to the control group but was less compared to group two meaning curcumin counters the increase of high-sensitivity-C-reactive protein levels induced by bisphenol A.
Malondialdehyde concentration was highest in the group treated with only bisphenol A. This is in line with the study by Aboul Khadrawy et al.28 It was a little lower in the group treated with both bisphenol A and curcumin which means curcumin counters the effect bisphenol A has on the level of the free radical malondialdehyde. The curcumin group and the control group had the lowest malondialdehyde concentration though that of curcumin was a little less compared to the control group. This is in line with the study by Alizadeh et al.,29 which showed curcumin plays a huge role in decreasing malodialdehyde levels.
The group administered only bisphenol A had the lowest concentration of glutathione which agrees with the study by Amjad Rahman et al.,30 that bisphenol A decreases the levels of glutathione in the blood. Glutathione concentration was highest in the curcumin group, followed by the control group. This agrees with the study by Alizadeh and Kheioruri29 which means it prevents oxidative stress by increasing the release of the free radical scavenger glutathione.31 The group treated with both bisphenol A and curcumin showed glutathione levels close to that of the control group which means curcumin is a powerful antioxidant and neutralizes harm caused by free radicals by increasing the release of enzymatic antioxidants. The results of the biochemical analysis indicate that bisphenol A induces oxidative damage by inducing oxidative stress and reduces cellular antioxidant capacity as reported by Aboul Khadrawy et al.,28 The present research agrees with Peng et al.,11 that curcumin reduces inflammation via its anti-oxidant activity by reducing the reactive oxidation species by increasing the release of anti-oxidant enzymes.
The group administered with bisphenol A only showed shrunken and ruptured villi, reduced number of goblet cells with degenerative changes compared to the control group. This is in line with the study by Ambreen et al., and Sharma et al.32,33 The group administered both bisphenol A and curcumin showed intact villi, bigger than that of the bisphenol A group but smaller compared to the control group. Therefore, this agrees with the study by Liu et al.,34 that curcumin ameliorates villous atrophy and villi loss. The group administered with curcumin showed optimum size of villi with very fine epithelium, hence curcumin mediates cytoprotection against oxidative stress.
The bisphenol A group showed mild amyloid plaques indicating, abnormal buildup of protein in tissues eventually leading to organ dysfunction and death as reported by Baker and Rice,35 The control, curcumin group and the group administered with both bisphenol A and curcumin showed no amyloidoses. The present research shows that curcumin inhibits the formation of amyloid protein aggregates as reported by Stefani et al.36–40
Bisphenol A has cytotoxic effect on intestinal cytoarchitecture and curcumin holds potential as a protective agent against BPA-induced cytotoxicity in the jejunum. Curcumin is a phenomenal antioxidant and also gives cytoprotection to organs.
None.
The authors declare that there are no conflicts of interest.

©2025 Elijah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.