MOJ
eISSN: 2574-9722


Review Article Volume 7 Issue 3
1Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria
2Department of General Surgery, Hospital Böblingen, Bunsenstraße 120, Böblingen, Germany
3Independent researcher
4Department of General Surgery, Medical University of Vienna, Vienna, Austria
Correspondence: Irene Kührer, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria
Received: April 28, 2022 | Published: May 18, 2022
Citation: Kührer I, Benz S, Maksimovic M, et al. Consensus recommendation (from the 11th conference of the European federation for colorectal cancer): Adjuvant chemotherapy after neoadjuvant chemoradiotherapy for rectal cancer and TME surgery. MOJ Biol Med. 2022;7(3):76-80. DOI: 10.15406/mojbm.2022.07.00169
Chemoradio therapy followed by total mesorectal excision is the standard of care for rectal cancer. Although locoregional recurrence and survival have improved, distant recurrence has not. Adjuvant chemotherapy might prevent distant metastases, however, its use for patients with rectal cancer treated with preoperative radiochemotherapy and surgery is largely debated. Available data do not support its routine use in these settings, unlike in colon cancer where adjuvant therapy role is well established. According to ESMO Guidelines, adjuvant chemotherapy after preoperative radiochemo therapy with postoperative histology stage III and high-risk stage II can be considered (level of evidence is lower than in colon cancer). These consensus recommendations have been developed based on the review of current evidence and expert opinions, and are expected to assist in selecting subgroups of patients that could benefit from adjuvant chemotherapy after neoadjuvant radio chemotherapy and TME surgery and choosing chemotherapeutic agent in different post-operative scenarios.
Keywords: adjuvant chemotherapy, rectal cancer, TME surgery
Colon and rectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer death worldwide.1 It accounted for about 1.4million new cases and almost 700 000 deaths in 2012, with an expected increase to more than 2.2million new cases and 1.1 million cancer deaths by 2030, representing a substantial public health problem.2 The American Cancer Society estimates 44,180 new cases of rectal cancer will occur in 2019, predominantly in males.3 The incidence of CRC exhibits considerable global variations and is closely associated to the features of the so-called western lifestyle.4 More than two thirds of all cases and about 60% of all deaths occur in regions with a high or very high human development index (HDI).2 On the other hand, there is a declining trend of CRC incidence in countries with the highest HDI, such as USA; Australia, New Zealand and several Western European countries, probably reflecting early detection and prevention, as well as improvements in therapy.1 As for the demographic features, men are more often affected than women and incidence substantially increases with age.4
The standard of care for rectal cancer (RC) is preoperative neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME). However, despite the improved local control after TME surgery, the rate of distant metastases remains relatively unaffected over the last time periods.5 Adjuvant chemotherapy (CT) might prevent distant metastases by eliminating circulating tumour cells and micrometastases. Both the European Society for Medical Oncology’s and the National Comprehensive Cancer Network’s Clinical Practice Guidelines recommend adjuvant CT for stage III and for high-risk stage II RC patients, who present at least one of the following negative prognostic factors: contiguity infiltration of neighbouring organs (T4b), grading G3, inadequate number of lymph nodes, analysed vascular, lymphatic and/or perineural invasion, clinical presentation with perforation or occlusion. Guided by these recommendations and driven by the facts such as high risk of CRC recurrence after CRT and surgery, proven benefits of adjuvant CT in patients with colon cancer (CC), and high risk for metastases in circumferential rectal margin (CRM) positive or extramural vascular invasion positive CRC, many oncologists will decide to prescribe adjuvant CTs to their patients.6,7 However, there is an ongoing debate about the actual benefit of adjuvant CT, particularly in patients who achieved a pathologic complete response (pCR) to neoadjuvant CRT and resection. Therefore, the current practice of adjuvant CTs for CRC is not based on solid evidence and thus varies around the world.6
Specific subgroups of patients that could benefit from adjuvant CT after neoadjuvant CRT for RC and TME surgery were discussed in the consensus session so called “Paradigm Changes of Adjuvant Therapies in Rectal Cancer” as a part of the 11th International Congress of European Federation for Colorectal Cancer held on April 24-27, 2019 in Vienna, Austria. An international panel of five expertsurgeons and oncologists was chaired by Prof. Stefan Benz. Before the panel discussion, a review of relevant literature was presented focusing on management of unexpected high tumour stage after primary resection for rectal cancer, arguments in favour and against adjuvant CT after surgery. Some of the available evidences were then corroborated by presenting various patients subgroups responses from the German registry data. The panelists, which included Prof. Stefan Benz, Prof. Eric Ruiller, Prof. Dirk Arnold, Prof. Robert Glynne-Jones, Prof. Irene Kührer and Prof. Bela Teleky, then jointly discussed benefits of adjuvant CT and the choice of chemotherapeutic agent in different post-operative scenarios below.
“Pro”
Before the TME surgical procedure was widely employed, a pooled analysis of three American randomized adjuvant RC trials that included 3,791 patients indicated that surgery alone and surgery in combination with radiotherapy (RT) had worse survival out comes when compared with CT.7 The QUASAR trial from the same period showed an improvement in overall survival for patients with stage II CRC treated with adjuvant fluorouracil (5-FU) and leucovorin. However, in this study, none of the patients had been treated with CT prior to surgery and only 21% of them with RC or both the CC and RC received preoperative RT.8
Benefits of adjuvant CTs in CRC have also been documented in the “new era”, when the TME surgery and preoperative RT and CRT took off. A phase III Chronicle trial compared 18 weeks of adjuvant capecitabine and oxaliplatin (XELOX)CT with no adjuvant treatment in patients who underwent resection following neoadjuvant 5-FU-based CRT. Unfortunately, the study was closed prematurely due to poor accrual, enrolling only 113 of the projected 800 patients. Even though there was an improvement in disease-free survival (DFS) for adjuvant XELOX compared to observation (78% vs. 71%, hazard ratio (HR)=0.80), the finding was not statistically significant, given the small number of patients and consequent low power.9
Multicenter prospective PROCTOR/SCRIPT trials examined patients with stage II or III RC who underwent short-course RT or CT followed by TME and were randomly assigned to observation versus postoperative 5-FU/leucovorin (PROCTOR), or observation versus postoperative capecitabine (SCRIPT) regimens. Once again, trials did not reach their full accrual. In a combined analysis of both trials, after a median follow-up of 5 years, overall survival (OS) was similar between observation and adjuvant CT groups. Still, an important finding was the HR of 0.80 for DFS in CT groups (p=0.13).10
A meta-analysis of individual patient data from four randomized controlled trials (PROCTOR/SCRIPT, EORTC 22921, Chronicle and I-CNR-RT) revealed that adjuvant 5-FU-based CT did not improve OS, DSF or distant recurrences in CRC patients. However, a subgroup analysis of patients with a tumour localized 10 to 15cm above the anal verge, showed significantly better DSF with adjuvant CT (HR 0.59) and fewer distant recurrences (HR 0.61).11 It is noteworthy that this meta-analysis had some significant limitations. The primary endpoint of interest was OS, and not DFS like in individual studies. In all modern studies of adjuvant treatment for CRC, DFS is regarded as a more suitable primary endpoint because OS is heavily affected by subsequent treatments and a relatively long follow-up is needed to observe an effect of new treatment strategies. In addition, there are no good explanations why half of the patients were excluded from the meta-analysis (eg, 538 of 1011 patients were excluded in the EORTC 22921 trial). Furthermore, it remains unclear why patients with ypTNM0 and ypTNM1 disease were excluded; as such cases were included in the two largest trials in the meta-analysis and possibly may benefit from adjuvant treatment the most. There is also a large heterogeneity in randomization time points and dose intensity and regimen of CT.12
In the EORTC trial 22921, the addition of 5-FU-based CT preoperatively or post operatively to the patients who had received preoperative RT exhibited no beneficial effect on survival.13 The latest update of this trial also confirmed no evidence that adjuvant CT improves 10-year OS or DFS.14 A very possible reason for such poor outcomes of this study might be the administration of “Mayo-like” adjuvant regimen, which is not generally accepted for the treatment of metastatic disease nor as an adjuvant therapy in CC.
Newer regimens are based on the addition of oxaliplatin to the 5-FU-based adjuvant therapies such as FOLFOX (fluorouracil, leucovorin, and oxaliplatin) or CAPOX (capecitabine plus oxaliplatin). Direct evidence for the benefit of these regimens in improving DFS in patients with resected RC after neoadjuvant CRT have beenprovided by MOSAIC, NSABP-C07 and XELOXA trials (HR=0.80 for all 3trials).15-17 The German CAO/ARO/AIO-04 study also showed that adding oxaliplatin both to 5-FU-based neoadjuvant CRT and to adjuvant CT significantly improved DFS of patients with RC stages cT3–4 or cN1–2.18 On the other hand, NSABP R04 and PETACC6 trials did not demonstrate the same benefits.19,20
Moreover, results of a recent meta-analysis demonstrated that the addition of oxaliplatin to 5-FU-based neoadjuvant CRT significantly improved DFS for a locally advanced RC, decreased distant metastases, and increased CR rate compared to 5-FU-based regimens alone. No significant differences were noted in local recurrence rates, R0 resection rates and CRM status between the two groups.21
Support for the intensification of CT with adjuvant FOLFOX regimen is also provided by the multicentre randomized phase II ADORE study, which exhibited superiority of the FOLFOX regimen in patients with curatively resected pathological stage II (ypT3–4N0) or III (ypTanyN1–2) RC after preoperative CRT when compared with adjuvant 5-FU plus leucovorin treatment. Adjuvant FOLFOX improved 3-year DFS in patients with advanced disease (HR=0.66), which is in contrast with the results of the EORTC 22921 trial which showed that patients who did not respond to preoperative CRT (ypT3–4) did not benefit from adjuvant 5-FU and leucovorin.22 In the latest update of the ADORE trial presented at the 2018 American Society of Clinical Oncology annual meeting, after a follow-up of 74 months, the FOLFOX group still had a significantly higher DFS(68% vs. 57%). A subgroup analysis found that patients with ypN2 stage and those with minimally regressed tumours had most benefit from FOLFOX therapy.23
Overall, benefits from adjuvant CTs exist, however they are not crystal clearly displayed. While the data from different trials are inconsistent, the majority of them show some benefits. An improvement in DFS of approximately 20% has been observed in several trials. Most of the trials that showed no benefits had several limitations. Probably the most solid evidence of the adjuvant CTs’ efficacy came from the ADORE study. If the adjuvant CT is indicated, then its intensity should be stratified according to many different factors (e.g. pathological TNM stage, T substage, CRM status, proportion of involved lymph nodes etc.). Thus, it seems that the lack of level 1a evidence should not hinder oncologists from treating patients at their best interest.
“Contra”
RC recurs after CRT and surgery in approximately 30% of cases. However, neither adjuvant 5-FU alone nor FOLFOX are able to reduce micro-metastases and subsequently distant recurrences of RC. In an already mentioned meta-analysis, Breugom et al. recorded 415 distant recurrences and found no significant benefit of adjuvant CT compared with observation. At 5 years, the cumulative incidence for distant metastases was 36.5% in the observation group compared to 35.5% in the CT group. A study also found no heterogeneity in treatment effect on distant metastases between the four trials.11
It is very important to bear in mind that if adjuvant CTs are successful in treating patients with CC, that may not necessarily mean that they will have the same effect on RC. There are substantial etiological, anatomical and clinical differences between CC and RC. For example, low physical activity, high body mass index, predominant animal-based diet and alcohol intake are known risk factors for CC, but not for RC. The prevalence of genetic mutations or mutation patterns and hereditary cancer types also vary between CC and RC. Anatomically, the colon and rectum have different blood supply, drainage and innervation. Mechanisms for developing recurrences and metastases differ too. Furthermore, preoperative RT leads to the various changes of the treated RC. This might be a very possible reason why postoperative 5-FU-based CTs are more successful for CC patients (especially proximal/right-sided tumours), compared to RC patients.24
The only predictive factor in CC for the benefit of CT is node positivity (pN+). Consequently, pN2 is a stronger predictive factor than pN1, whereas insufficient nodes is a surrogate for stage III cancer. In addition, deficient mismatch repair (dMMR) and high microsatellite instability (MSI-H) statuses have been shown to have a negative predictive value for adjuvant 5-FU therapy alone in stage IICC. The best evidence comes from the analysis of adjuvant trials in the ACCENT database, in which no benefit and even a trend toward worse survival was observed in patients with MSI-H stage II tumors treated with adjuvant 5-FU.However, in the same study, patients with stage III tumors showed a significant survival benefit from adjuvant 5-FU-based treatment regardless of MSI status.25,26 Nevertheless, there are also no evidence for the benefits of oxaliplatin adjuvant CTs in stage II CC.27,28
In RC, ypN+ cannot be considered as a predictive factor after RT or CRT. Although a pooled analysis by Gunderson et al. showed benefits of 5-FU after RC surgery, patients included in this study did not receive preoperative RT nor CRT.7 In fact, the EORTC 22921 trial revealedno survival differences between patients who received and those who did not receive adjuvant 5-FU after neoadjuvant RT/CRT and surgery.14 Breugom et al. also showed that there is a total nodal discordance between CC patients who have not received RT and RC patients who have received RT, thus concluding that ypN2 is not a predictive factor of adjuvant 5-FU or oxaliplatin therapy.11
In a retrospective analysis, which included series of stage II/III RC patients treated with short-course preoperative RT and TME, those who received adjuvant CT had much better survival outcomes. However, this was an “outcome by outcome” analysis prone to bias. Patients who completed the treatment were younger, healthier, with better performance status, without surgical morbidities and co-morbidities and hence had a better prognosis than those who failed to receive the treatment.29 Therefore, any association between compliance and outcome does not necessarily mean that it was the actual treatment received which is associated with better or worse outcomes.
Another study indicates that postoperative complications (POCs) following CRC resection are associated with adverse oncologic outcomes and overall survival, which generally deteriorate proportionally to the increase in the Clavien–Dindo grading. Data indicate that POCs occurring in stage III patients or classified as Clavien–Dindo IV are also associated with reduced rates of CT use, caused by omissions, delays, or discontinuations of adjuvant CT in CRC. An existence of perioperative morbidity (e.g. diabetes, heart disease) rather than the number of poorly performed surgeries could be a possible reason for such unfavourable post operative outcomes.30
To predict local recurrence, distant metastases, and survival for patients with locally advanced RC treated with long-course CRT and surgery and to allow a selection of patients who may benefit most from postoperative adjuvant CT, Valentini et al. developed nomogram prediction tools. In one of the nomograms developed to predict metastasis rates, predictors such as ypN stage, ypT stage, surgery procedure, and adjuvant CT were combined. The addition of adjuvant CT improved the distant metastases figures in the metastasis nomogram, but the benefits were small and insignificant.31
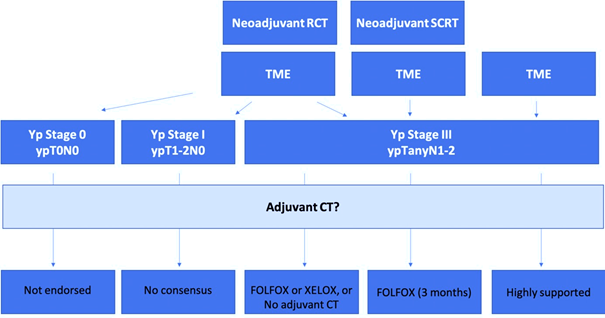
Figure 1 Proposed consensus algorithm.
FOLFOX, leucovorin/fluorouracil/oxaliplatin; RCT, radiochemotherapy; SCRT, short course radiotherapy; TME, total mesorectal excision; Yp, pathological stage after (chemo)radiotherapy; XELOX, capecitabine plus oxaliplatin.
Although, both CAO/ARO/AIO-04 and ADORE trials showed that adjuvant CTs improved DFS of RC patients, they have failed to show improvements in OS and had some substantial limitations. In CAO/ARO/AIO-04 trial, most of the patients who had benefits were younger than 60years, with better performance status, whereas in patients older than 70years no clear benefits have been shown. In ADORE trial, the median age of all participants was 54 and only 17% of all patients were older than 65years.18,22
In most of the trials that found no benefits of 5-FU therapy alone, but did find the benefits of FOLFOX for the treatment of RC patients, rates of d-MMR/MSI-H were between 1-10%. Subgroup analyses of these trials demonstrated that patients younger than 50 years had much higher rates of d-MMR/MSI-H, most probably because of the increased incidence of Lynch syndrome in younger patients. Evidently, 5-FU does not work for patients with high d-MMR/MSI-H rates.32-34
An interesting finding from a long-term follow-up of ADORE trial is the difference of about 12% between DFS after 6-years of adjuvant CTs with 5-FU alone versus FOLFOX which was not translated into the benefit of OS. This can be explained by the fact that ADORE trial selected out a cohort of patients with metastatic disease (ypN+ and ypT3) who were initially resistant to 5-FU and then treated with 5-FU-based CRT which made their eventual residual cells even more resistant to further adjuvant 5-FU-based CT.23
Overall, resistance to CRT confers a high-risk and neither of the currently available adjuvant CTs (5-FU nor FOLFOX) is able to reduce micro metastases after CRT. There are no significant randomized trials and meta-analyses showing a clear benefit of adjuvant CTs following CRT in OS. There are also no trials comparing 5-FU versus control, or XELOX versus control showing improvement in DFS. Only one, small phase II ADORE trial showed promising results of adjuvant CTs by improving DFS rates, however in this study, patients were predominantly young and with better performance statuses.
Data from the German Clinical Cancer Registries
An analysis of the data from 34 German clinical cancer registries collected in the period from 2000 to 2016 and covering approximately 28% of all diagnosed CRCs in Germany was performed in an effort to present various patients subgroups responses to different therapeutic approaches, as well as to present the current trends in practice. Of 334,057 patients with primary diagnosis of RC stage II to III, 18,897patients had complete datasets. After excluding patients over 75years of age, those operated on weekends and those with fatal outcome following the surgery, a cohort of 13,568patients was analyzed. Multivariate analysis of the relationship between age, sex, CRC localization, stage, grading and number of affected lymph nodes was performed.
The results have shown a notable increase in the use of preoperative RCT with or without adjuvant CT in the observed period of time. The number of patients with no additional therapy besides surgery has remained stable, while exclusive application of either adjuvant CT or RT has decreased remarkably over the years.35 The 10-year DFS was highest in patients who received neoadjuvant RCT either with or without adjuvant CT, and lowest in patients with no adjuvant therapy at all. The effect of adjuvant CT following the preoperative RCT was least important in patients with pCR, but in patients with postoperative cancer stage I it significantly improved the DFS. However, this positive effect of adjuvant CT decreased in patients with postoperative cancer stage II/III, and especially in those with the higher number of affected lymph nodes. Similarly, in patients with postoperative cancer stage III who received no neoadjuvant therapy, neither adjuvant RCT nor adjuvant CT exhibited any effect on DFS.35
There are a number of limitations to these results, including the fact that these were non-randomized registry data, the involvement of CRM was not known and neither the type of CT nor the dosage of RT was specified. Still, they do offer some initial conclusions as well as a good starting point for creating a consensus on the use of adjuvant CT after neoadjuvant RCT in different stages of RC.
Case 1: Patients with postoperative pCR (ypT0N0) after neoadjuvant CRT
Recommendation: The routine use of adjuvant CT is not endorsed. Patients with ypCR appear to have an excellent prognosis regardless of use of adjuvant CT. The potential benefit of adjuvant CT in these patients would appear to be rather small, if it exists at all.
Case 2: Patients with postoperative pathological stage III (ypTanyN2) RC after neoadjuvant CRT
Recommendation: Adjuvant 5-FU CT alone is not adequate for nodal-positive disease. The panelists agreed that either adjuvant short-course FOLFOX of XELOX could be a treatment option for patients with locally advanced RC after fluoropyrimidine-based preoperative CRT, or no adjuvant CT at all.
Case 3:Patients with postoperative pathological stage I (ypT1-2N0) RC after neoadjuvant CRT
Recommendation: Consensus is not reached. Three panelists would not recommend adjuvant treatment at all, since the prognosis of such patients is still very good. One expert would recommend adjuvant CT with fluoropyrimidine alone. The opinion of another expert is that immunotherapy may be beneficial for these patients, unlike adjuvant CT.
Case 4:Patients with postoperative pathological stage III (ypTanyN1-2) RCafter short-course neoadjuvant RT (5x5 Gy)
Recommendation: The clinical evidence of the benefits of the adjuvant CT in this scenario is largely lacking. The PROCTOR-SCRIPT trial could not demonstrate a significant benefit of adjuvant CT with fluoropyrimidine monotherapy regarding OS, DFS, and recurrence rates after preoperative CRT and TME surgery in ypTNM stage II and III RC patients. Despite this, the panelists recommend adjuvant CT with FOLFOX for 3 months in this setting. Possibly, upper rectal tumours are biologically more similar to low sigmoid tumours that benefit from adjuvant CT.
Case 5: Patients with postoperative pathological stage III (ypTanyN1-2) RC without neoadjuvant CRT
Recommendation: Adjuvant CT in postoperative, node-positive RC without neoadjuvant CRT is highly supported.
The concept of total neoadjuvant treatment in rectal cancer was proven indifferent trials. The RAPIDO trial shows that in patients with high-risk locally advanced rectal cancer short-course RT followed by 18-weeks CT before surgery decreases the probability of disease-related treatment failure.36 Dose reduction of CT occurred in 201 (44%) of 460patients in the experimental group. Reasons for stopping CT were toxicity. In 65 (14%) patients, CT was to be stopped, because of Grade 3 toxicity.
The total neoadjuvant approach was also under investigation in the PRODIGE 23 trial (preoperative CT before CRT, followed by TME and adjuvant CT).37 The initial results showed significantly increased 3-year DFS, metastasis-free survival, and pCR rate compared with CRT followed by TME but in this trial adjuvant therapy was added. Future studies will show which patients really benefit from intensified induction therapy or traditional consolidation.
None.
The authors declared no have conflict interest for the study.

©2022 Kührer, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.