MOJ
eISSN: 2573-2951


Research Article Volume 4 Issue 2
1Department of Pharmacology and Pharmaceutical Chemistry, Rajarambapu College of Pharmacy, India
2Department of Physiology, Krishna Institute of Medical Sciences University, India
Correspondence: Rahul Shivaji Adnaik, Assistant Professor, Department of Pharmacology and Pharmaceutical Chemistry, Rajarambapu College of Pharmacy, Kasegaon, Maharashtra, Tal. Walwa, Dist. Sangli 415404, India, Tel +91 8600009720
Received: November 04, 2017 | Published: December 12, 2017
Citation: Adnaik RS, Gavarkar PS, Mohite SK, et al. The neuroprotective efficacy of Lagenaria vulgaris extract against oxidative stress in rat hippocampus. MOJ Bioequiv Availab. 2017;4(2):267-269. DOI: 10.15406/mojbb.2017.04.00069
The neuroprotective potential of ethanolic extract of Lagenaria vulgaris (EELV) seeds against Oxidative Stress in Rat Hippocampus was evaluated to ascertain the validity of folkloric claims. The neuroprotective potential was evaluated through oxidative stress induced by sodium azide in the hippocampus of rats. Brain homogenates were pre–incubated for 30 minutes with 10mg/mL EELV and then incubated for 60 minutes with 5mM sodium azide and were examined for oxidative markers viz., thiobarbituric acid reactive substances (TBARS), catalase (CAT), superoxide dismutase (SOD) since their levels are altered on exposure to sodium azide. The results indicated that the levels of TBARS, CAT activity were enhanced and SOD activity was reduced by sodium azide. EELV prohibited the changes in TBARS, CAT and SOD. Cell viability was evaluated by the 3(4,5–dimethylthiazol–2–yl)–2,5–diphenyl tetrazolium bromide (MTT), and it’s showed no change in MTT reduction by Sodium azide. However EELV increased MTT reduction in the presence or absence of sodium azide. Hence it can be concluded that EELV has potential antioxidant and neuroprotective activity in the rat hippocampal homogenate which may be due to the presence of flavonoids.
Keywords: Lagenaria vulgaris, antioxidants, oxidative stress, neuroprotective, sodium azide
EELV, ethanolic extract of lagenaria vulgaris; TBARS, thiobarbituric acid reactive substances; CAT, catalase; SOD, superoxide dismutase; AD, alzheimer’s disease; Ach, acetylcholine; CPCSEA, purpose of control and supervision of experiments on animals; ATP, adenosine triphosphate
Alzheimer’s disease (AD) is a progressive and neurodegenerative disorder affecting the elderly population.1 AD is the commonest form of dementia and affects approximately 60 million people worldwide and 5.2 millions in US alone. AD is associated with selective death of cholinergic neurons, specifically in areas of the brain that mediate learning, memory and cognition functions, resulting in deficiencies in the neurotransmitter, acetylcholine (ACh). Inhibition of acetylcholinesterase (AChE), the enzyme responsible for the hydrolysis of ACh, elevates the levels of ACh and consequently, improves the disease severity.2 Moreover, senile plaques composed of Aβ-peptides are the hallmark of AD.3 Although, the initial source of oxidative stress in AD is still unclear, multiple studies have associated oxidative stress and free radical damage to the etiology of AD. It is reported that brains with AD have high levels of H2O2, hydroxyl and oxygen radicals that peroxidize membrane lipids and oxidize proteins producing drastic cellular damage.4,5 Restricted treatment remedies accentuate the importance of developing effective strategies for diminishing AD. In light of this Lagenaria vulgaris (L. vulgaris) from Cucurbitaceae family was investigated for the presence of neuroprotective compounds that could inhibit AChE enzyme or decrease the neurotoxicity of oxidative damage. This can help in finding alternative strategies for the treatment of neurodegerative disorders.
Plant material and preparation of extracts
The seeds of L. vulgaris were obtained from a local market of Kolhapur, Maharashtra (India), which were taxonomically identified and authenticated by Botanical Survey of India, Pune. A voucher specimen was submitted at Institute's herbarium department for future reference. The seeds of L. vulgaris were coarsely powdered (500g) and the powder material (200g) was extracted using 70% ethanol and the menstrum collected was concentrated till dry to obtain dark brown colored semisolid mass of EELV (Yield 58%).
Determination of the total flavonoid content in the extracts
Aluminium chloride method was used for flavonoid determination.6 Quercetin was used as standard and flavonoid contents were measured as quercetin equivalent. For this purpose, the calibration curve of quercetin was drawn. 1ml of standard or extract solution (20, 40, 60, 80,100mg/l) was taken into 10ml volumetric flask, containing 4ml of distilled water. 0.3ml of 5% NaNO2 added to the flask. After 5min, 0.3 ml 10% AlCl3 was added to the mixture. At the 6th min add 2ml of 1M NaOH was added and volume made up to 10ml with distilled water. The absorbance was noted at 510nm using UV-Visible spectrophotometer
Experimental animals
Young Wistar rats (10-days-old) were used in the present study. Animals were housed in polypropylene cages in groups of 5-6 animals per cage under laboratory conditions (alternating light and dark cycle of 12h each). Animals had free access to food and water. They were maintained at 22 ± 2°C, on a 12-h light/12-h dark cycle, with free access to food and water. The experimental procedures were conducted according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India
Tissue preparation
The animals were euthanized by decapitation, and the brain was rapidly excised on a Petri dish placed on ice. The hippocampus was dissected, and kept chilled until homogenization which was performed in 1.5% KCl, using a ground glass type Potter-Elvejhem homogenizer. The homogenates were centrifuged at 800 × g for 10 minutes at 4°C; the pellet was discarded and the supernatants were kept at -70°C until the determinations.
Incubation
The hippocampus homogenates were pre-incubated at 37°C for 30 minutes with EELV (10mg/mL) and then incubated for 60 minutes at 37°C, with 5mM sodium azide and the continued presence of EELV (Figure 1).
Measurement of oxidative stress
Thiobarbituric acid reactive substances (TBARS) measurement: For the TBARS assay, trichloroacetic acid (10% w/v) was added to the homogenate to precipitate proteins and to acidify samples.7 This mixture was then centrifuged (1000 × g, 3 minutes). The protein-free sample was extracted and TBA (0.67% w/v) was added to the reaction medium. Tubes were placed in a water bath (100°C) for 30 minutes. Absorbance was read at 535nm in a spectrophotometer (SHIMADZU UV-1800). Commercially available malondialdehyde was used as a standard. Results were expressed as mmoL/mg protein.
Determination of antioxidant enzyme activities: Superoxide dismutase (SOD) activity, expressed as USOD/mg protein, was based on the decrease in the rate of autocatalytic adrenochrome formation at 480nm.8 Catalase (CAT) activity was determined by following the decrease in hydrogen peroxide (H2O2) absorbance at 240nm and expressed as CAT/mg protein.9
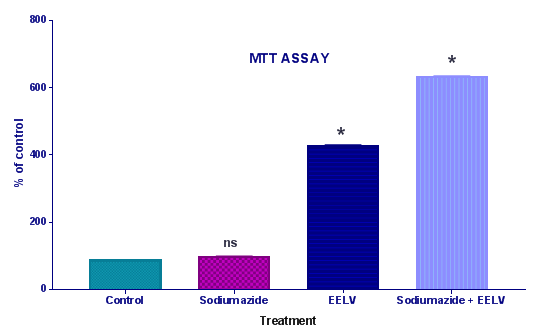
Cell viability assays by methylthiotetrazol salt method (MTT): The viability assay was performed by the colorimetric 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method.10 Immediately after pre-incubation and incubation of the homogenates, 5mg/mL MTT was added to the medium, followed by incubation at 37°C for 30min. The formazan product generated during the incubation was measured at 490 and 630nm (Figure 2).
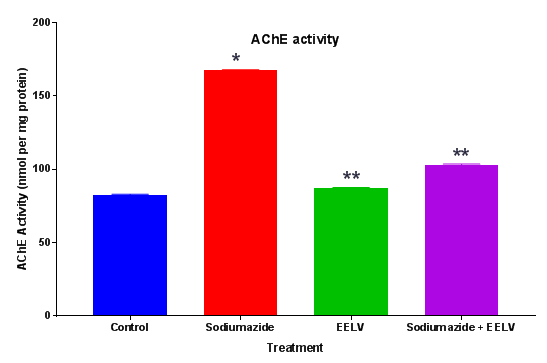
Estimation of acetylcholinesterase (AChE) levels: AChE is a marker of loss of cholinergic neurons in the forebrain. The whole brain AChE activity was measured by the method of Ellman et al.11 The enzymatic activity in the supernatant was expressed as nmol per mg protein11 (Figure 3).
Protein determination: Protein concentration was determined by the method of Lowry et al. 12 using bovine serum albumin as standard.12
Statistical analysis: Data from the experiments were analyzed statistically by two-way analysis of variance (ANOVA) followed by the Tukey test. Values of P < 0.05 were considered significant. All analyses were carried out using the Statistical Package software Graph pad prism version no: 5.0.
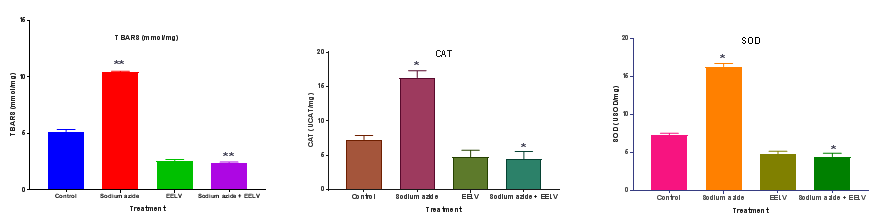
The amount of total flavonoid was determined the Quercetin reagent. Quercetin was used as a standard compound and the total flavonoid were expressed as mg/g Quercetin equivalent using the standard curve equation: y = 0.119 x, R2= 0.998, Where y is absorbance at 510nm and x is total flavonoid content in the extracts. The total flavonoid content measured by AlCl3 reagent in terms of Quercetin acid equivalent was found to be 42.6 ± 0.23mg/g. Antioxidant properties of various natural products are assayed using a stressor that causes injury or cell death. Hence, in the present study, sodium azide treatment was used as an in vitro experimental model for oxidative damage. Sodium azide acts by inhibiting complex IV (cytochrome oxidase) in the electron transport chain leading to hypoxia, by inhibiting the formation of adenosine triphosphate (ATP), and promoting an increase in reactive species, including superoxide anion and hydrogen peroxide, which may cause oxidative stress and cell death. TBARS is used to evaluate lipid and protein damage. Table 1 demonstrates that sodium azide increased TBARS. EELV prevented the increase in TBARS. These results are in conformity with the observations of Souza et al.13 The enzymatic antioxidant defenses (CAT and SOD) were evaluated, in view of the fact that they are the most important enzymes of the antioxidant defense. These enzymes represent the first barrier against reactive species and are vital for cell survival. The enzyme SOD catalyzes the dismutation of superoxide anion into oxygen and hydrogen peroxide. The enzyme CAT catalyzes the reduction of hydrogen peroxide to water and molecular oxygen. In the present study, it was established that sodium azide increased CAT activity (Table 1) and reduced SOD activity in rat hippocampal homogenate, and that these alterations were prevented by EELV. Similar observations are reported by Souza et al.13
Treatment |
TBARS (mmol/mg) |
CAT (UCAT/mg) |
SOD (USOD/mg) |
Control |
05.16 ± 0.186 |
07.30 ± 0.062 |
7.89 ± 0.834 |
Sodium Azide |
10.42 ± 0.086** |
16.31 ± 1.012* |
4.65 ± 1.174* |
EELV |
02.56 ± 0.138 |
4.80 ± 0.962 |
8.68 ± 1.132 |
Sodium Azide + EELV |
02.41 ± 0.054** |
4.48 ± 1.117* |
8.28 ± 1.101* |
Table 1 Effect of ethanolic extract of L. vulgaris extract (EELV) and sodium azide on antioxidant enzymes in rat hippocampal homogenate.
Further, the treatment with sodium azide impaired the learning and memory and increases the levels of brain AChE.14 Effect of EELV on brain AChE level in sodium azide treated rats showed a significant rise in brain AChE activity in comparison to control animals. Administration of EELV to sodium azide treated rats showed a significant decline in brain AChE activity in comparison to sodium azide treated control group animals. Sodium azide induced Oxidative damage might lead to neural cell death. MTT assay was used to evaluate the effect of sodium azide and EELV on the viability of the homogenates. In the present study sodium azide did not alter MTT reduction levels in the rat hippocampal homogenate. However, EELV augmented MTT reduction in the presence or absence of sodium azide, demonstrating that the extract enhanced cell viability and showed some beneficial effect against cell death. Furthermore, sodium azide significantly compromises the non-enzymatic (sulfhydryl) and enzymatic (CAT and SOD) antioxidant defenses; thus increasing the levels of reactive species, although it did not induce cell death in the rat hippocampal homogenate. Hence, there was an imbalance between pro-oxidants and antioxidants leading to oxidative stress. Additionally, EELV also prevented the oxidative damage induced by sodium azide.
EELV has potential antioxidant and neuroprotective activity in the rat hippocampal homogenate. Thus, it can be concluded that the intake of Flavonoid–rich foods could reduce, delay or even inhibit the progression of oxidative stress related disorders.
Authors are thankful to Dr. CS Magdum, Principal, Rajarambapu College of Pharmacy for providing the research facilities to carry out the research work.
The author declares no conflict of interest.

©2017 Adnaik, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.