MOJ
eISSN: 2573-2951


Research Article Volume 3 Issue 3
SNDT Women's University, India
Correspondence: Pratima Tatke, Professor of Pharm Chem, CU Shah College of Pharmacy, SNDT Womens University, Juhu Campus, Juhu Tara Road Santacruz West, Mumbai 400 049, India, Tel 9920685857
Received: January 31, 2017 | Published: May 10, 2017
Citation: Oberoi K, Tatke P. Piperine enhances the bioavailability of secnidazole in rats. MOJ Bioequiv Availab. 2017;3(3):76-81. DOI: 10.15406/mojbb.2017.03.00037
Secnidazole is rapidly absorbed after oral administration and it has a bioavailability of about 80% when given in high dose of about 2 g. The high dose of Secnidazole causes many side effects even with a single dose such as dizziness, abdominal pain, urticaria, glossitis, stomatitis, taste disturbances, paresthesias and more severe side effects include leucopenia and Secnidazole–induced acute pancreatitis. Hence the present study aims to increase the bioavailability of secnidazole by a bioenhancer, piperine, in order to reduce the therapeutic dose of secnidazole, thus decreasing its side effects. Analytical and bioanalytical methods were developed and validated for simultaneous estimation of secnidazole and piperine by HPLC. Pharmacokinetic study was carried out in rats for secnidazole alone and secnidazole – piperine combination. The results revealed that the dose of Secnidazole can be reduced up to 1/3rd of its original dose, which will lead to better patient compliance.
Keywords: secnidazole, piperine, bioavailability, bioenhancer
BA, bioavailability; BV, bacterial vaginosis; TLC, thin layer chromatography; HPLC, high performance liquid chromatography; LOD, limit of detection; LOQ, limit of quantification; LLOQ, lower limit of quantification; ULOQ, upper limit of quantification; QCs, quality control samples; CV, coefficient of variation; AUC, area under curve
Bioavailability (BA) is defined as the rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action. For drug products that are not intended to be absorbed into the bloodstream, bioavailability may be assessed by measurements intended to reflect the rate and extent to which the active ingredient or active moiety becomes available at the site of action.1
Some drugs have poor bioavailability and hence take a longer time to act. These drugs therefore do not pass the phase 1 of the clinical trials as these do not meet the necessary criteria of having the maximum bioavailability and hence show a delayed onset of action. In such cases of bioavailability issues, one of the optimal solutions can be bioenhancement of that drug with a suitable bioenhancer to augment its bioavailabilty and hence a dose reduction. There are some drugs, however, which have a moderate bioavailability but only when given in higher doses. One such drug is Secnidazole.
Secnidazole is a 5-nitroimidazole. It has a spectrum of activity against anaerobic micro-organisms and it is particularly effective in the treatment of amoebiasis, giardiasis, trichomoniasis and bacterial vaginosis. Secnidazole is rapidly absorbed after oral administration and it has a bioavailability of about 80%.2
The major advantages of Secnidazole are as follows:3,4
In patients with intestinal amoebiasis or giardiasis, clinical or parasistological cure rates of 80 to 100% are achieved after treatment with a 2g dose of Secnidazole. This high dose of Secnidazole causes a lot of side effects even with a single dose.
The symptomatic side effects produced by Secnidazole include dizziness, abdominal pain, urticaria, glossitis, stomatitis, taste disturbances, paresthesias. There are cases where the therapy has been withdrawn after a single administration due to these effects. If they become severe, they can be treated symptomatically.5
The severe or irreversible side effects of Secnidazole, which give rise to further complications include leucopenia. Secnidazole-induced acute pancreatitis cases have also been reported after a single dose administration.6
This high dose of Secnidazole, which on single administration causes side effects, hence, makes the rationale for our current study involving combination of Secnidazole along with bioavailability enhancer like piperine. This can result in reduction of dose of Secnidazole, thus reducing its side effects, and hence better patient compliance.
Secnidazole has a biosimilar Solosec, which is a potent, next-generation, investigational 5 nitroimidazole antibiotic with enhanced pharmacokinetic properties anticipated to be the first and only single-dose oral therapy approved for the treatment of bacterial vaginosis (BV). In clinical trials Solosec™ demonstrated efficacy for the treatment of BV with only a single, oral, two-gram dose, with excellent safety, tolerability and adherence.7 The summary of the work is given below:
Isolation and purification of piperine
Piperine was isolated from the fruits of Piper nigrum with a percentage yield of 1.6996% w/w. The purity and identity of the isolated piperine was confirmed by chromatographic analysis (TLC and HPLC) by comparing with standard piperine. TLC analysis revealed similar RF values for isolated and standard piperine. From HPLC analysis, the peak areas obtained for isolated and standard piperine were used to calculate the % purity. The % purity of isolated piperine was found to be 89.0699% by HPLC studies. Various chemical studies such as studies such as colour, odour, solubility, melting point, and chemical test with conc. sulphuric acid showed results similar to that of standard piperine, thus indicating the identity of isolated piperine. Spectral studies such as UV spectroscopy was also performed which confirmed the identity of isolated piperine. Hence, the purity and identity of isolated piperine was confirmed.
Analytical method development and validation
A simple, sensitive and reliable HPLC analytical method was developed and validated for simultaneous estimation of Secnidazole and piperine. This method can be helpful in simultaneous estimation of Secnidazole and piperine from formulation containing Secnidazole and piperine. The method was validated as per ICH guidelines. The conditions for the developed and validated HPLC method are summarized as follows:
Development of HPLC parameters
Optimization of method
Selection of column: C18 column was used for the analysis as it is rugged, retentive, and widely used for analysis. Also, it is a most commonly used type of column due to efficiency in terms of selectivity, lifetime and operating pressure.
Selection of mobile phase: From the various trials performed, the mobile phase consisting of acetonitrile and water resulted into a sharp peak with better resolution for simultaneous estimation of Secnidazole and piperine. Hence, acetonitrile: water in the ratio 95:5 was optimized as the mobile phase for the analysis.
Selection of pH: In the trials, it was observed that pH adjustment causes peak fronting and the pH adjustment affects the reproducibility of the results. However, the present study used a mobile phase of acetonitrile and water (95:5v/v), which did not need pH adjustment and or a phosphate buffer, which leads to marked wear in the chromatographic column and compartments of the HPLC. Therefore, the method which is used is easier, precise and cost effective.
Selection of flow rate: The analysis was carried out at 0.5,0.8 and 1ml/min. Considering the system suitability parameters (number of theoretical plates and peak symmetry) which were better when run at 0.5ml/min, hence this was optimized as flow rate.
Selection of detector wavelength: The λmax of Secnidazole and piperine are 310nm and 340nm respectively. An overlay was carried out for Secnidazole and piperine by UV spectroscopy and the overlay wavelength was found to be 327nm. Both Secnidazole and piperine were detected at this wavelength. Hence, the λmax for the simultaneous estimation of Secnidazole and piperine was fixed at 327nm (Figure 1).
Selection of column temperature: The analysis was carried out at ambient temperature (Table 1 & Figure 2).
The validation parameters were as follows
Selectivity: Selectivity of an analytical method is the ability to measure selectively the analyte of interest without interferences from blank. A chromatogram was recorded for the blank to check for interference. Mobile phase [ACN: Water (95:5)] was used as blank. There was no interference of blank at the retention time of analyte peak.
S.no |
Parameters |
Method |
1 |
Column Specification |
Hypersil, ODS C-18 Column having Dimensions of 250mm x 4.6mm and i.d. 10μ Particle Size |
2 |
Mobile Phase |
Acetonitrile: Water (95:5v/v) |
3 |
pH |
No pH Adjustment |
4 |
Flow Rate (ml/min) |
0.5 |
5 |
Run Time (min) |
10 |
6 |
Column Temperature (°C) |
Ambient Temperature |
7 |
Volume of Injection Loop (μl) |
20 |
8 |
Detection Wavelength (nm) |
327 |
9 |
Detector |
UV Detector |
10 |
Type of Elution |
Isocratic |
11 |
Retention Time (min) |
|
Secnidazole |
5.683 |
|
Piperine |
6.834 |
Table 1The following table gives the optimized conditions for bioanalytical development for simultaneous estimation of Secnidazole and piperine.
Linearity: The concentrations for both Secnidazole and piperine used for calibration were in the range 2-10μg/ml. The response was determined to be linear over the range of 2-10μg/ml for both Secnidazole and piperine. The solutions were injected into HPLC system. Each of the concentration was injected in triplicate to get reproducible response. The run time was 10 min and the peak areas were measured. The calibration curve was plotted as concentration of the respective drug versus the response (area) at each level. The proposed method was evaluated by its correlation coefficient and intercept value calculated by statistical study. They were represented by the linear regression equation. Data from the regression line itself was helpful to provide mathematical estimates of the degree of linearity. The y-intercept, slope of the regression line and residual sum of squares of correlation coefficient are reported in the results.
Range: The range was derived from linearity studies as 2-10μg/ml. It was established by confirming that the analytical procedure provided an acceptable degree of linearity, accuracy and precision when applied to samples containing amounts of analyte within or at the extremes of this specified range.
Accuracy: Accuracy was established across the specified range of the analytical procedure. To check the accuracy of the proposed method, recovery studies were carried out according to ICH guidelines by applying the standard addition method to known amount of Secnidazole and piperine corresponding to 50,100 and 150%.
Precision: The inter-day and intra-day precision of the method was determined. Three concentration levels were analysed in triplicate corresponding to 50,100 and 150 %. The area of drug peaks and percentage RSD were calculated.
Limit of detection (LOD) and limit of quantification (LOQ): Limit of detection was determined based on the standard deviation of the response and the slope. The detection limit (LOD) may be expressed as:
LOD = 3.3 (σ/S)
LOQ = 10 (σ/S)
Where, σ = the standard deviation of the response,
S = the slope of the calibration curve
The slope S may be estimated from the calibration curve of the analyte. The estimate of σ was done by calculating standard deviation of y-intercepts of regression lines.
Robustness: The robustness of the method was studied, during method development, by small but deliberate variations in flow rate and detection wavelength. One factor at a time was changed at a concentration level of 10μg/ml of both Secnidazole and piperine, to study the effect on the retention time and peak area of the drugs.
System suitability testing: System suitability testing is an integral part of many analytical procedures. It includes resolution, number of theoretical plates, and tailing factor as some of the main system suitability parameters.
Validation results
Bioanalytical Method Development and Validation
Development of HPLC Parameters
Optimization of method
Selection of mobile phase: The mobile phase used was the same as that used for the analytical method development. The mobile phase used was ACN: water in the ratio 95:5v/v. At these conditions, no interference due to plasma components was observed with that of analyte peaks.
Selection of pH: From the analytical method, it was seen that no pH adjustment is required. Hence, no pH adjustment was carried out.
Selection of flow rate: The analysis was carried out at different flow rates such as 0.5, 0.8 and 1ml/min. The system suitability parameters (number of theoretical plates and peak symmetry) were found to be better at a flow rate of 0.5ml/min. Hence, flow rate of 0.5ml/min was selected.
Selection of detector wavelength: The λmax of Secnidazole and piperine are 310 nm and 340 nm respectively. The overlay wavelength was observed at 327 nm. Hence, 327 nm was selected as the detection wavelength.
Bioanalytical method validation (As per FDA guidelines)
The parameters for the bioanalytical method development and validation included selectivity, accuracy, precision, recovery, calibration curve, sensitivity, and stability of analyte in spiked samples.
Selectivity: Selectivity is the ability of an analytical method to differentiate and quantify the analyte in the presence of other components in the sample. The procedure is designed to demonstrate the capability of the chosen method to separate Secnidazole and piperine against the components of the plasma matrix. Six samples of blank plasma were analysed, in order to evaluate the method selectivity.
Linearity: The linearity assessment is a procedure designed to measure the capability of both sample preparation and the chromatographic methods, to produce results related in a linear way to the concentrations of the analytes in the plasma samples. From each spiked plasma sample there were taken three aliquots of 1ml (except for LLOQ, where 6 aliquots of 1ml were taken). On these aliquots, was applied the sample preparation method previously described. The calibration curve readings consisted of six non-zero samples covering the expected range, including LLOQ. The calibration curve included concentration range of 0.1, 0.5, 1, 1.5, 2.5 and 5µg/ml.
Lower limit of auantification (LLOQ): The lowest concentration of standard on the calibration curve is accepted as the LLOQ if the analyte response at the LLOQ is at least five times the response compared to blank response. The lower limit of quantification (LLOQ) was 0.1μg/ml. At this concentration, analyte peak (response) was identifiable, discrete, and reproducible, and the back-calculated concentration has accuracy within 20% of the nominal concentration and precision that did not exceed 20% of the CV.
Upper limit of quantification (ULOQ): The highest concentration of standard in the calibration curve was 5μg/ml. Hence, Upper Limit of Quantification was 5μg/ml. Analyte peak (response was reproducible and the back calculated concentration has accuracy within 20% of the nominal concentration and precision that did not exceed 20% of the CV.
Quality control samples (QCs): At least three concentrations of QCs in duplicate was incorporated into each run as follows: one near three times the LLOQ (low QC), one in the midrange (middle QC), and one approaching the high end (high QC) of the range of the expected study concentrations. The three QC samples that were selected for validation included 0.5μg/ ml (low QC), 1.5μg/ ml (middle QC) and 5μg/ ml (high QC).
Extraction efficiency: The recoveryof an analyte in an assay is the detector response obtained from an amount of the analyte added to and extracted from the biological matrix, compared to the detector response obtained for the true concentration of the analyte in solvent. Recovery pertains to the extraction efficiency of an analytical method within the limits of variability. Recovery of the analyte need not be 100%, but the extent of recovery of an analyte and of the internal standard should be consistent, precise, and reproducible. Recovery experiments were performed by comparing the analytical results for extracted samples at three concentrations (low, medium, and high) with unextracted standards that represent 100% recovery.
Accuracy: The accuracy of an analytical method describes the closeness of mean test results obtained by the method to the actual value (concentration) of the analyte. Accuracy was determined by replicate analysis of samples containing known amounts of the analyte (i.e. QCs). Accuracy was measured using five determinations per concentration. The three concentrations included 0.5, 1.5 and 4.5μg/ ml.
Acceptance criteria: The mean value should be within 15% of the actual value except at LLOQ, where it should not deviate by more than 20%. The deviation of the mean from the true value serves as measure of the accuracy.
Precision: The precision of an analytical method describes the closeness of individual measures of an analyte when the procedure is applied repeatedly to multiple aliquots of a single homogeneous volume of biological matrix. Precision was measured using five determinations per concentration for the three QC samples which included 0.5, 1.5 and 5μg/ml. The precision determined at each concentration level did not exceed 15% of the coefficient of variation (CV) except for the LLOQ, where it did not exceed 20% of the CV.
Acceptance criteria: The mean value should be within 15% of the actual value except at LLOQ, where it should not deviate by more than 20%. The deviation of the mean from the true value serves as measure of the precision.
Sensitivity: Sensitivityis defined as the lowest analyte concentration that can be measured with acceptable accuracy and precision (i.e. LLOQ). The precision and accuracy studies were carried out at 0.1 μg/ml (LLOQ) and found to be within the acceptance criteria. Hence, the method was found to be sensitive.
Stability: Freeze thaw stability of the spiked quality control samples was determined during three freeze thaw cycles stored at below -20 ± 5°C. Stability was assessed by comparing against the freshly spiked quality control samples. The concentration changes related to the nominal concentration were less than 15%, indicating no significant substance loss during the study (Table 1 & Figure 3).
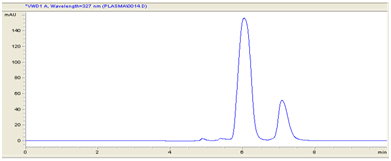
Figure 3 Chromatogram of simultaneous estimation of Secnidazole and piperine by bioanalytical method.
Bioanalytical validation results
Pharmacokinetic studies
Animals Housing and preparation for study: Albino rats (Wistar strain) were procured and housed in animal house. Animals were acclimatized to the experimental room for one week and conditioned at room temperature and natural photoperiods. Animals were randomly selected, marked to permit individual identification, and caged in polypropylene cages containing paddy husk as bedding with maximum of three animals in each cage and provided free access to standard food pellets as basal diet and water.
Preparation of doses and its administration: Secnidazole was dissolved in distilled water for oral administration. Doses were prepared freshly prior to administration. The drug was administered as a single dose by oral gavage using a suitable oral feeding needle.
Number of animals and dose levels: The rats were divided into three groups (6 rats per group) as detailed below:
The standard doses of Secnidazole and piperine were selected by calculating it from the human dose. It was calculated based on the total body surface area of the rat using the conversion factor 0.018. The human dose selected for Secnidazole and piperine were 2g and 2.2g for a 70kg human. Dose for Secnidazole was calculated as 0.0666mmol/kg and the dose for piperine was calculated as 0.1401mmol/kg (Table 2).
Drug |
Human Dose in mg/70 kg (a) |
Conversion Factor (b) |
Rat Dose in mg/kg (c) (c =a×b) |
Molecular Weight (d) |
Rat Dose in mmol/kg (c×d) |
Secnidazole |
2000 |
0.018 |
36 |
185.18 |
0.0666 |
Piperine |
2200 |
0.018 |
40 |
285 |
0.1401 |
Table 2 Calculation of rat dose from human dose in mmol/kg.
Methodology followed for the pharmacokinetic study: For bioavailability studies, Secnidazole alone and Secnidazole along with piperine was administered to each group of rats. The blood samples were collected from retro-orbital plexus at different time intervals that included 0, 1, 2, 4, 6, 8, 24 and 48 hours. Plasma was separated by centrifuging the blood sample at 2000rpm for 20 minutes. The concentration of Secnidazole in plasma was determined by HPLC method. Then, the pharmacokinetic parameters such as maximum plasma concentration (Cmax), time of maximum concentration observed (Tmax) and area under the plasma concentration time curve (AUC) were calculated and compared using statistical methods.
Analysis of plasma samples: The plasma samples were analysed by the developed and validated HPLC. Cmax and Tmax for both group II and group III were obtained by extrapolating the AUC value in the linearity graph of Secnidazole. Average area under the curve (AUC) was calculated and a graph of average AUC v/s time interval was plotted. The area under the curve (AUC) was calculated by applying trapezoidal rule (Table 3).
S.no |
Parameter |
Data |
|
Group II |
Group III |
||
1 |
AUC |
0.58785µg-h/ml |
1.1394µg-h/ml |
2 |
Cmax |
0.1273µg/ml |
0.3026µg/ml |
3 |
Tmax |
1h |
1h |
Table 3 The results of the pharmacokinetic study are summarized as follows.
The results from pharmacokinetic study indicated increase in AUC and Cmax for Secnidazole from 0. 58785µg-h/ml and 0.1273µg/ml in group II to 1.1394µg-h/ml and 0.3026µg/ml in group III respectively.
It is concluded from the Pharmacokinetic study that the Cmax and AUC of Secnidazole were found to be increased in the piperine administered group (Group III). On statistical analysis (Student t-test) of the results obtained, the p value was found to be less than 0.01. The increase in Cmax and AUC in group III was found to be statistically significant. Hence, the bioenhancement of Secnidazole was successfully achieved. Therefore, as per the aim of this study, the dose of Secnidazole can be reduced up to 1/3rd of its original dose, which will lead to better patient compliance.
None.
The author declares no conflict of interest.

©2017 Oberoi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.