MOJ
eISSN: 2573-2951


Review Article Volume 4 Issue 1
1Department of Botany, GC University, Pakistan
2Glass and Ceramics Research Center, Pakistan
3Department of Zoology, University of the Punjab, Pakistan
4Applied Chemistry Research Center, Pakistan
5Department of Statistics and Computer Sciences, University of Veterinary and Animal Sciences, Pakistan
6Govt. College Township Lahore, Pakistan
Correspondence: Phool Shahzadi, Glass and Ceramics Research Center, PCSIR, Labs. Complex Lahore, Pakistan
Received: June 17, 2017 | Published: October 25, 2017
Citation: Mehmood F, Shahzadi P, Khan ZUD, et al. In Vivo hepatoprotective and antidiabetic activities of essential oils from Boenninghausenia albiflora (Hook.) reichb. ex heynkh, of Pakistan. MOJ Bioequiv Availab. 2017;4(1):211-214. DOI: 10.15406/mojbb.2017.04.00060
In the present work essential oils extracted from different parts of a medicinally important shrub, Boenninghausenia albiflora, were evaluated for their hepatoprotective effect and hypoglycaemic activity. GC/MS analysis indicated that monoterpenes, sesquiterpenes, ketones, esters and alcohols dominated in all essential oils. The hepatoprotective potential of essential oils was studied in vivo in Wistar albino rats against carbon tetrachloride induced hepatotoxicity. The hepatoprotective activity was determined on the basis of their effects on biomarkers like aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), bilirubin and total protein (TP). All essential oils exhibited hepatoprotective activity in comparison with the standard drug. For antidiabetic activity two doses of essential oils i.e. 50 and 200mL/Kg body weight were introduced into alloxan induced diabetic rabbits. Although both doses were found effective to have hypoglycaemic effect but higher dose showed better control over diabetes, comparable to that of Glib (positive control). It can be concluded from the results that these essential oils can play significant role not only in the liver protection but also in management of diabetes.
Keywords: boenninghausenia albiflora, essential oil, hepatoprotective activity, hypoglycaemic effect, monoterpenes, sesquiterpenes, alcohols
AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; TP, bilirubin and total protein; HED, human equivalent dose; SGPT, glutamic pyruvic transaminase; SGOT, glutamic oxaloacetic transaminas
Although there are many plant families in Pakistan including essential oil rich plants, but Rutaceae represented by 11 genera and 27 species, many of which have been naturalized here being cultivated and hybridized for edible, medicinal and ornamental purposes. Many genera especially wild plants with essential oils and commercially viable new hybrid still need to be analyzed thoroughly regarding their ethnopharmacological significance. Boenninghausenia albiflora is a monotypic genus found in temperate Himalayas. Commonly found in the N. of Pakistan (Hazara and Murree Hills) at 200-3000m, growing in shady forest. The plant has an unpleasant smell and the roots have a spicy fragrance. Locally called “Pissu. Mar” or flea killer, its leaves when crushed emit a strong foetid smell and used as a flea powder. As a result of indiscriminate use of antimicrobial drugs in the treatment of infectious diseases, microorganisms have developed resistance to many antibiotics.1 There is a need to develop alternative antimicrobial drugs. One approach is to screen local medicinal plants, which represent a rich source of novel antimicrobial agents. Boenninghausenia albiflora belonging to the family Rutaceae and is well known for its medicinal properties in traditional system of medicine. In ethnobotanical literature, the aerial as well as the root part has been described as an antiseptic while leaf part has been used to apply on cuts and wounds whereas root powder is being used as antiseptic.2 Some-times its juice is also being given in vomiting and dysentery, while some workers also reported this plant to have flea repellent,3 as well as calcium blocking activity.4 The present study was carried out to investigate the chemical composition of its essential oils, antioxidant potential and the antimicrobial properties. As no previous records on these aspects of this plant could be found in the literature in Pakistan, the present study claims some useful results.
Experimental
Plant material was obtained from its natural habitat, identified and authenticated by a botanist in Dr. Sultan herbarium, GC University, Lahore. The respective plant parts were separated and subjected to hydro-distillation for about four hours. The essential oils obtained thus were dried over anhydrous sodium sulphate and stored in dark colored glass bottle at temperature of about 4°C.
GC-MS analyses were performed using a Shimadzu GCMS-QP2010A system in EI mode (70eV) equipped with an injector at 250 °C and a DB-5MS column. Samples were injected at 250 °C with a split ratio of 50/50. Injection volume was 1µl and electronic pressure programming was used to maintain a constant flow (0.67ml/min) of the helium as carrier gas. The oven temperature was programmed from 100 °C (4min) to 250 °C at a rate of 2 °C/min and held at this temperature for 2min. The mass spectrometer was set to scan the mass range 40amu to 600amu with ion source temperature 200 °C and interface temperature 250 °C. Analyses were performed in triplicate with a blank run after every analysis. The resulting data was processed using Shimadzu Lab Solution GCMS Post-run Analysis software. The relative apparent percentage of each compound and of their classes was determined by area normalization method. Identification of compounds was made by comparing with the mass fragmentation pattern of the reported data in NIST 147 and NIST 27 libraries.
Determination of hepatoprotective activity
Preparation of animal house for rats and feeding routine: Male Wistar albino rats, Rattus norvegicus with weight 250 ± 20g, taken from the Animal House, University of Veterinary and Animal sciences, Lahore, were held in standard cages placed in the animal house at Zoology Dept., University of the Punjab, Quaid-i-Azam Campus, Lahore, for 7 days for acclimatisation. Standard pellet diet and tap water were supplied ad libitum.
Liver toxicity induction: Liver toxicity was induced by CCl4. It’s toxicity has been characterized extensively and a link is recognised between its metabolism, generation of free radicals, and peroxidative decomposition of cytoplasmic membrane structural lipids.4 Additionally, CCl4-induced toxicity causes centrilobular necrosis and leakage of cellular enzymes into blood stream.
Solvents used as dilutors: Tween 20 was used as dilutor for the essential oils of the plant parts under study.
Grouping of animals: After acclimatization for 7 days all rats were distributed into 6 groups (A, B, C, D, E and F of five rats each. Group A acted as untreated uncompromised, control and was not given any treatment. Group B also treated as control was given was administered with 50µl of Tween 20 dissolved in equal amount of water. Each groups viz. C, D and E of rats received single dose of essential oil, i.e. 50mL, that corresponds to the human equivalent dose (HED) as per following formula: HED = rat dose /kg (weight of rat in kg/weight of human in kg) 0.33 , dissolved in 50µl of Tween 20 by stainless feeding needle for fourteen days. Group R acted as positive control group. This group was administered with locally available drug, Silymarin. This drug was used @100mg/kg diluted in Tween 20 also.
Collection of blood: Four hour after the last dose, a single dose of CCl4 (1.5mg/kg in ratio of 1:1 olive oil v/v) was administered orally to animals of all the groups except controls (groups A and B). After 24h, blood sample of each of the animals was obtained from the heart in a centrifuge tube. The blood samples were allowed to coagulate for 30 min at 37 °C and then serum was separated by centrifugation at 2500rpm at 10 °C for 10 min that was stored at -80 °C until analyzed. A portion of liver of each of the animals was preserved in neutral buffered formalin (10%) for histology.
Biochemical analysis for determination of liver function biomarkers: The serum samples were analyzed for the determination of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), bilirubin and total protein (TP). The analysis was carried out in Biochemistry Laboratory of Mayo Hospital Lahore using RIELE Semi Automated Chemistry Analyser, Photometer5010 V5+, Robert Riele Gmbh & Co KG (Germany) Software version 5.1, Documentation Version 1.2008.
Assessment of liver toxicity: To assess hepatic toxicity, serum samples of each of the animals of group A to F were subjected to biochemical analysis for the estimation of liver function markers and the results were compared between these groups.
Evaluation of antidiabetic activity
Preparation of animal house for rabbits: Rabbits Oryctolagus cuniculus weighing > 750g ± 20 and aged 8-12 months were kept in the animal house earthen pots were kept in animal house for rabbits. Animal house was twenty feet in length, ten feet in width. Standard conditions were maintained in the animal house regarding its temperature, relative humidity along with dark and light cycle.
Preparation of alloxan diabetic rabbits: The rabbits were made diabetic by injecting intravenously alloxan monohydrate 150mg/Kg body weight. This dose permanently destroys the beta cells of pancreas and produces diabetes mellitus. Eight days after injection of the alloxan monohydrate, blood glucose of all the surviving rabbits was determined by checking direct glucose level by glucometer. Water and diet were available to the animals throughout the treatment period. After eight days rabbits confirmed diabetic with blood glucose level from 200-500mg/100ml were selected for the evaluation for antidiabetic activity.
Experimental plan: After acclimatization for 7 days all rabbits were distributed into 5 groups (A, B, C, D, E, F, G and H of five rabbits each. The group A acted as control and was not given any treatment, but 10 ml water. The group B and C received an application of 50 and 200mL/kg body weight, of EO from leaves B. albiflora diluted in CMC 1% and water. The group D and E received an application of 50 and 200mL/kg body weight, of EO from stem of B. albiflora diluted in CMC 1% and water. The group F and G received an application of 50 and 200mL/kg body weight, of EO from root of B. albiflora diluted in CMC 1% and water. Group H acts as positive control group. This group was administered with locally available drug, Glibenclamide (Glib.). This drug was used in 0.5mg/ml in concentration diluted also in 10 ml of 1% CMC. The glucose level was recorded after 5, 10, and 24 hours daily.
Acute oral toxicity: Acute oral toxicity of all essential oils was carried out adopting “up and down procedure” as per given in the OECD guidelines, Anonymous.5 Four groups each of rabbits and rats were formed separately, having 5 animals each. Weights of overnight fasting animals were taken and an oral dose of each essential oil @ 2000mg/kg was given to one animal of each of the groups. Food was stopped for further 3hrs and the animals were observed, once during the first 30min and then for 24h at regular intervals with special attention in the first 4h. All animals survived; the same dose was given to the additional 4 animals of each group, and observations were made for 14 days as per OECD guidelines.
Statistical analysis: One-way ANOVA and Probit-Regression tests were applied, using SPSS 13.0 (statistical soft ware) on the data for statistical analysis to draw conclusions.
Hypoglaecimic effect
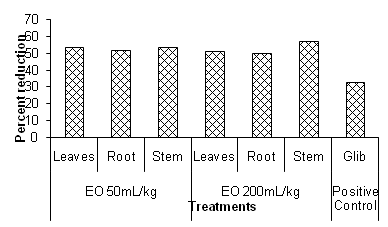
STZ-induced b-cell death in the pancreas is due to the alkylation of DNA, hence producing hyperglycaemia, Elsner et al.6 Both low and high concentrations of essential oils have exhibited hypoglaecemic effect. Immediate drop in the blood glucose levels can be seen. Effect of high dose of essential oil is about almost equal to the low dose. However results of both doses i.e. 50 and 200mL/kg of essential oils (Table 1) are far better than the effect of Glib (positive control) Figure 1. All these findings suggest that essential oils may be acting through some mechanism to improve the receptor-responsiveness to insulin causing increased sugar uptake by the tissue. It is very difficult to comment on the exact mechanism of hypoglycaemic activity of essential oils, since the study was not designed accordingly. However b-linalool and alcohols present in B. albiflora essential oils could be responsible for its hypoglyceamic activity by virtue of their antioxidant property, Rajanarayana et al.7
Treatments |
Glucose Level Alloxan Induced Rabbits mg/dl |
Glucose Level mg/dl (Mean±SEM) |
|||
5hrs |
10hrs |
24hrs |
|||
EO 50mL/kg |
|||||
B. albiflora Leaves |
225±0.98 |
214±1.30 |
143.6±1.07 |
120.6±0.67 |
|
B. albiflora Root |
230±0.23 |
217.5±1.15 |
151.2±0.58 |
119.2±0.86 |
|
B. albiflora Stem |
229±0.41 |
216±1.33 |
147±0.70 |
123±0.68 |
|
EO 200mL/kg |
|||||
B. albiflora Leaves |
230±0.40 |
211±0.70 |
135.2±0.86 |
118.2±1.24 |
|
B. albiflora Root |
221±0.62 |
200.8±0.86 |
140.4±0.81 |
110.8±0.86 |
|
B. albiflora Stem |
225±0.92 |
214.6±0.59 |
152.6±0.81 |
129±1.24 |
|
Glib 0.5mg/ml |
342±0.58 |
137±0.70 |
131.6±0.50 |
111.6±0.50 |
|
Water |
222±0.33 |
225.6±0.50 |
233.6±0.50 |
242.4±0.92 |
|
Table 1 Effect of different doses of different EOs on oral glucose tolerance in Alloxan treated rabbits (n=5).
*Values expressed as means ± SEM
Hepatoprotective effect
The formation of free radicals in CCl4-induced liver stress results in a sharp increase in lipid peroxidation, which is not localized, hence, propagates between the cells to interact with phospholipids causing structural destructions.8 This destruction results in a loss of functional integrity of cell membrane, which causes the leakage of certain biomarkers into blood stream. These biomarkers, ALT, AST, ALP, LDH and bilirubin, are extensively being used to assess the liver function. ALT is also called serum glutamic pyruvic transaminase (SGPT) and is found in liver, kidney, heart and muscles. The content of this important biomarker is raised into blood stream in hepatic injury/toxicity because it leaks out from hepatocytes. Another marker, AST, also known as serum glutamic oxaloacetic transaminase (SGOT), is found in liver parenchyma cells, erythrocytes, cardiac tissue and skeletal muscles. In conditions involving hepatic disorders, level of AST is raised into blood stream. ALP is found in cell linings of biliary tract, the bones and placenta, and in liver damage or biliary obstruction, it enters into blood stream. LDH is another important enzyme responsible for inter conversion of pyruvate and lactate, and in oxidative stress its level is raised in blood. The level of these biomarkers in oils treated groups indicates that hepatocytes have shown resistance against free radicals of CCl4. Essential oils have shown hepatoprotectibe activity.9-14 In the present study, all essential oils have helped hepatocytes to resist against free radicals of CCl4, Table 2, indicates the effect of hepatotoxicity induced by CCl4. Figure 2 gives a picture of percent reduction in the raised values of biochemical markers. According to this maximum reduction in ALT was done by essential oil from roots and leaves followed by essential oil from stem. Maximum reduction in AST was done by essential oil from stem followed by leaves and roots Max reduction in Alkaline Phosphatases was by essential oil from root followed by oils from stem and leaves. As far as reduction in Bilirubin was concerned maximum reduction was shown by essential oil from roots and leaves followed by stem. Similarly reduction in total proteins was done by essential oil from root and stem that was upto 9.8% followed by 8.19% by essential oil from leaves. Maximum reduction in LDH value was done by essential oil from B. albiflora leaves followed by stem and roots. The essential oils not only offered protection but also caused start of regenerative process, which is a very encouraging effect in this regard. The essential oils have high content of flavonoids, alcohols and terpenes, which have contributed in hepatoprotective activity of the extracts through enhancing antioxidant mechanism of the animals. This claim is supported by Vimala et al.15 The administration of all essential oils for 14 days have protected the hepatocytes against CCl4-induced toxicity. Hence, the use of these essential oils as food supplement may be helpful to protect liver from oxidative damage in hepatitis and diseases involving long-term therapy.
Treatment |
ALT IU/L |
AST IU/L |
Alkaline Phosphatases IU/L |
Bilirubin (Direct) mg/dl |
Total Protien g/dl |
Lactate Dehydrogenase IU/L |
Control |
33.33±6.00 |
111.64±3.17 |
385±3.24 |
0.36±0.03 |
5.66±0.12 |
2039.33±3.11 |
Control(Tween 20) |
49.33±0.66 |
190±3.00 |
349±5.76 |
0.53±0.03 |
5.8±0.17 |
2095±2.10 |
CCl4 Induced |
183.33±1.68 |
272.66±3.89 |
519.66±0.18 |
0.5±0.03 |
6.1±0.31 |
4248±4.84 |
Boenninghausenia albiflora Roots |
23.33±1.20 |
174.33±3.84 |
268.33±2.02 |
0.36±0.03 |
5.5±0.05 |
2468.33±1.69 |
B. albiflora Stem |
46.66±0.88 |
141±5.5 |
344±1.93 |
0.4±0.00 |
5.5±0.08 |
2315.33±1.98 |
B. albiflora Leaves |
29.66±0.88 |
167.66±4.3 |
386.33±1.78 |
0.36±0.03 |
5.6±0.11 |
2042.66±4.60 |
Control (Silymarin) |
40±5.00 |
185.66±1.75 |
344±1.87 |
0.4±0.05 |
5.3±0.03 |
1710±2.22 |
Table 2 Biochemical Analyses to show the effect of Essential oils on CCl4 induced hepatotoxicity in rats (n=5).
*Values expressed as means ± SEM
None.
The author declares no conflict of interest.

©2017 Mehmood, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.