MOJ
eISSN: 2573-2951


Short Communication Volume 3 Issue 3
Department of Pediatrics, University of Debrecen, Hungary
Correspondence: Lajos Lakatos, Faculty of Medicine, Department of Pediatrics, University of Debrecen, Hungary
Received: April 04, 2017 | Published: May 11, 2017
Citation: Balla G, Pataki I, Lakatos L. A dangerous vitious circle of the neonatal brain in bilirubin-induced neurologic dysfunction (BIND). MOJ Bioequiv Availab. 2017;3(3):83-84. DOI: 10.15406/mojbb.2017.03.00038
On the basis of abundant research data and hypotheses the BIND is a neurodegenerative disease (ND) of immature brain caused by accumulation of free copper ions, unconjugated bilirubin (UCB), and UCB–Cu complex (as prooxidant), respectively, in the basal ganglia (BG) and other parts of the central nervous system (CNS) relevant to BIND. The main comorbidity is the hemolysis of neonatal red blood cells. During this process a great amount of heavy metals (mainly iron and copper) are liberated and producing reactive oxygen species (ROS). These elements may circulate in the bloodstream, and can pass through the immature blood–brain–barrier (BBB), finding entrance into the CNS. In addition, ROS contribute to increased BBB permeability creating a dangerous vitious circle in the neonatal brain, especially in the basal ganglia (BG). The beneficial effects of D–Penicillamine (D–PA) in neonates based on the capability of this drug to alter the NO system, and it is a strong antioxidant. Low molecular weight disulfides are the major products of D–PA metabolism in humans. The oxidation of D–PA in vivo may also important in the mode of action of the drug through simultaneous reduction of the ROS and reactive nitrogen species (RNS). Consequently, D–PA fulfils the criteria of a hybrid drug in the neonatal period by its ability to modulate both oxidative stress and NO pathway, and can be a neuroprotective agent in the pathophysiology of neurologic dysfunction.
Keywords: copper toxicity, bilirubin induced neurologic dysfunction, oxidative stress, copper bilirubin complex, d–Penicillamine in the neonatal period
BG, basal ganglia; ROS, reactive oxygen species; BBB, blood brain barrier; D–PA, d–penicillamine; UCB, unconjugated bilirubin; ND, neurodegenerative disease; CNS, central nervous system; RNS, reactive nitrogen species
The copper as a transitional metal is a powerful oxidant producing the highly damaging hydroxyl (OH-) radical. A literature review was aimed at assisting us (as pediatricians) to provide further understanding with bilateral symmetrical basal ganglia (BG) and thalamic lesions caused by Wilson's disease and BIND.1,2 Elevated levels of “free” or loosely bound metal ions can exert toxic effects, and in order to maintain homeostatic levels to protect the brain (especially the basal ganglia/BG) and retinal cells from their toxicity.3 In fact that appropriate mechanisms exist such as metal transporters, chaperones, and the presence of certain storage molecules that tightly bind metals to form nontoxic products, however, these mechanisms do not work in the neonatal period.4
Very wide-ranging studies have long been made on the possible biochemical transformations of unconjugated bilirubin (UCB). Particular attention has been paid to its photochemical and redox reactions but the relevant publications comprise only a very small proportion of those dealing with the molecular biochemistry of UCB and metals interactions. Bilirubin has a special affinity for the globus pallidus, the hippocampus, and the subthalamic nucleus because they are also target brain regions for divalent metal (Cu, Fe, Zn etc.) accumulation.5
Our new concept addresses the medical necessity of chelation therapy (with D-Penicillamine-/D-PA) in the neonatal period.6 As it is feasible that UCB molecule possesses particular affinity to copper stored in BG where copper-bilirubin complex can be formed together with the production of hydroxyl radical. Therefore, BIND is a neurological damage of immature brain caused by accumulation of free metals and UCB-Cu complex (as prooxidant) in the BG and other parts of central nervous system relevant to BIND. The main comorbidity is the hemolysis. During this process a great amount of heavy metals (mainly iron and copper) are liberated and producing ROS. These elements may circulate in the bloodstream, and can pass through the immature BBB, finding entrance into the CNS. In addition, ROS contribute to increased BBB permeability creating a dangerous vitious circle in the neonatal brain, especially in the BG. In strictly biological terms the two most important such metals are iron and copper.7,8 The beneficial effects of D-PA in neonates based on the capability of this drug to alter the NO system, and it is a strong antioxidant. Low molecular weight disulfides are the major products of D-PA metabolism in humans.9 The oxidation of D-PA in vivo may also important in the mode of action of the drug through simultaneous reduction of the ROS and reactive nitrogen species (RNS). Consequently, D-PA fulfills the criteria of a hybrid drug in the neonatal period by its ability to modulate both oxidative stress and NO pathway, and can be a neuroprotective agent in the pathophysiology of neurologic dysfunction10 (Figure 1).
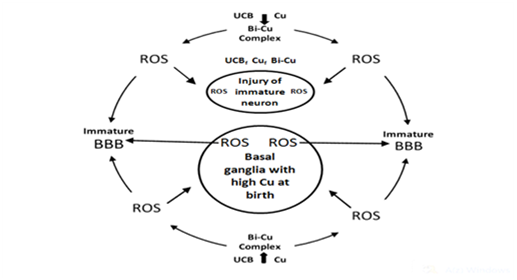
We hope that our concept will help answer some of the unsolved questions and concerns ocurred in the etiology and pathomechanisms of BIND and other neurodegenerative/neurodevelopmental disorders. The beneficial neuropharmacological actions of metal-targeted (chelating) agents most likely arise from local metal redistribution rather than from massive metal removal.11,12 The chelation therapy for non-metal overload indications continues to be investigated. Our present article address the medical necessity of the use of a chelating agent (D-PA) in the prevention or treatment of neonatal brain injuries.
None.
The author declares no conflict of interest.

©2017 Balla, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.