MOJ
eISSN: 2573-2951


Research Article Volume 3 Issue 2
1Zabol University of Medical Sciences, Iran
2Department of Biochemistry, Sussex University, UK
3Department of History of Medical Sciences, Iran University of Medical Sciences, Iran
Correspondence: Mohammad Ali Darbandi, Analytical Research Laboratory, School of pharmacy, Zabol University of Medical Sciences, Zabol, Iran, Tel +98 542 22535278, Fax +98 5422253528
Received: September 04, 2016 | Published: March 23, 2017
Citation: Zadeh SN, Rajabnezhad S, Zandkarimi M, et al. Mucoadhesive microspheres of chitosan and polyvinyl alcohol as a carrier for intranasal delivery of insulin: in vitro and in vivo studies. MOJ Bioequiv Availab. 2017;3(2):39-45. DOI: 10.15406/mojbb.2017.03.00030
The aim of this study was to investigate the capabilities of chitosan microspheres as drug carrier system in co-formulation with polyvinyl alcohol (PVA), to improve the systemic absorption of intranasal insulin delivery.
Insulin loaded microspheres was developed from varying ratio of chitosan solutions along with polyvinyl alcohol (PVA) as additive polymer using spray drying method. Different formulations were developed and morphological studies of the optimized formulas showed that the size range of spherical shaped particulate matters existed from 200 nm to 2 μm. It was clearly observed that physicochemical properties of the microspheres were extensively affected by changing the concentration ratio of the two polymeric materials. In vitro studies of insulin release pattern was performed in various time intervals up to 24 h. It was evident that microspheres made up of chitosan showed initial burst release but slower release as the experiment continued. Microspheres made up of combination of the aforementioned two polymers had instant, sharp and burst drug release. Surprisingly there was no absorption after intranasal delivery of chitosan-PVA microspheres in groups of rats comparing to formulated chitosan microspheres having profound absorption due to their smaller particle size, slower drug release rate and better mucoadhesive properties. Therefore, significant reduction in the plasma blood glucose level for chitosan based optimized formulation was seen right after 4.5 hours compared with control group. The aim of the present study was to fabricate an appropriate application of polymeric microspheres for intranasal delivery of insulin using a novel optimized formulation based on industrial level spray drying technique and to realize the possible barriers in scale up process of its large scale production, considering the effectiveness of polyvinyl alcohol (PVA) and chitosan to increase mucoadhesivness, gelling ability and ultimately effective release behavior pattern of insulin. According to this study, the combination of the polymers used and the mean particle size of formulated microspheres were found to be key factors in insulin drug release resulting for further enhancement of insulin absorption via intranasal route of delivery.
Keywords: intranasal delivery, microspheres, blood glucose level, absorption enhancement, mucoadhesive polymers, chitosan, polyvinyl alcohol
PVA, polyvinyl alcohol; IV, intravenous; IM, intramuscular; GI, gastrointestinal; MW, molecular weight; SEM, scanning electron microscopy; AUC, area under curve; MDT, measurement of mean dissolution time; SD, standard deviation.
Diabetic mellitus is a chronic disease that appears as result of absolute or partial deprivation of insulin. It constitutes a group of metabolic disorders which is common in hyperglycaemic patients. It would be regarded as an imminent cause of disease attributed to more than 25% of the blindness compared to non-diabetic patients. Insulin is a peptide hormone which regulates the metabolism of carbohydrates and fats by promoting the absorption rate of glucose level within the cells. It has been shown that absorption of peptides and proteins by the oral route is quite low or even negligible based owing to their molecular weight, polarity and lipophilicity of the drugs, enzymatic degradation and instability etc. Hence administration of insulin like macromolecules are generally carried out by parenteral therapy i.e., subcutaneous or intramuscular routes of delivery rather than oral route.1,2
Invasiveness of the Intravenous (IV) or intramuscular (IM) injections as well as the least patient compliance compelled scientists either to modify the previously existed dosage forms which are already in the market to prevent them from gastrointestinal (GI) degradations or to explore other possible route for drug delivery. Apart from oral or injection therapies, various other means for insulin delivery has been already investigated including, transdermal, buccal, nasal, pulmonary, ocular and rectal delivery.3 Intranasal route has traditionally been practiced for centuries in the treatment of various local diseases such as nasal congestion, sinusitis, allergic, rhinitis and infectious illnesses. In the last few decades, treatment of disease once a rapid onset of action is desired have been more emphasized such as migraine, severe pains, menopausal symptoms and asthma like attacks.
Intranasal delivery is a safe route of drug administration providing vast surface area for drug to be absorbed with higher degree of patient compliance compared to parenteral routes. Large surface area and presence of wide mucociliary network like structures are key parameters for intranasal drug delivery. They directly influence the entrance of drug molecules into the systemic circulation after being absorbed depending on lipophicity of drug molecular weight (Mw). Furthermore, avoidance of hepatic first pass metabolism makes it a preferred route for the delivery of small drug molecules but not basically the macromolecules as the permeability and degradability are rate-limiting steps in their systemic absorptions.
Feasibility of intranasal route for delivery of peptides and proteins has already been evaluated as mentioned before, due to the perceived less demanding barriers to peptides and proteins transport across the nasal epithelium as compared to the oral mucosa and nasal cavity comprising lower enzymatic content, higher leakiness of the membrane and high vascularity and no hepatic first pass metabolism But still there are few nasal peptide based products which have appeared on the market e.g. calcitonin (Miacalcin®, Fortical®), desmopressin (Desmospray®), nafarelin (Synarel®) buserelin (Suprecur®) and oxytocin (Syntocinon®).4,5
These products do not contain absorption enhancers and hence would show low bioavailability but are effective enough due to the low plasma levels needed to obtain a therapeutic effect.
As indicated above, one of the demerits of intranasal administration of peptides and proteins is their low bioavailability.6 This can be attributed to their intrinsic chemical structure as well as their physicochemical properties such as hydrophilicity, high Mw and intrinsic instability in the presence of enzymes. Owing to their physicochemical properties, peptides and proteins are expected to pass the nasal membrane via the paracellular pathways more specifically via the tight junctions. However, the diameter of the tight junctions in the nasal cavity are ranged from 3.9-8.4Å7 and hence the transport through these junctions would be limited or considered to be negligible for larger drug molecules, such as insulin with a structural diameter of 26.8Å in the monomeric form.8 It has already been shown by Costantino et al.9 that permeation enhancers were able to open the tight junctions in the nasal mucosa to about 10–15 times higher, approaching a maximal diameter in the order of 15 nm.9 The application of absorption enhancers were often studied in mucoadhesive like formulations in the form of liquids, gelling systems, nanoparticles and microspheres by few authors to better approach the transport of peptides and proteins across the nasal mucosa.10-16 Of special interest has been the use of chitosan as an absorption enhancer and a bioadhesive materials acting by opening the tight junctions of nasal mucosa to overcome the high degree effect of the mucociliary clearance by increasing the residence time of the formulation in the nasal cavity.17
Chitosan, a natural linear polycationic, biocompatible, biodegradable and mucoadhesive polymer derives from alkaline deacetylation of chitin of several Mw (10kDa–2000kDa) with varying degrees of deacetylation (40-98%). The inherent characteristics of the chitosan determines physicochemical properties of the polymer which are key for its effectiveness as an absorption enhancer as well as being a mucoadhesive material. Chitosan is positively charged in acidic pH, where it forms a water soluble salt. The positive charges interact with the sialic acid in the mucus and makes it vulnerable to disrupt the tight junctions by translocation of the membrane and cytocylic proteins into the cells cytosol, effectively increasing the systemic absorption of proteins and peptides.18 Its apparent pKa is dependent on the degree of deacetylation, but generally is in the order of 6.3.17 Application of chitosan microspheres for nasal delivery for drug molecules such as morphine,19 zolmitriptan,20 verapamil,22 metoclopramide,22 insulin,23-25 goserelin26 and human growth hormone13 using different production methods i.e., spray drying20 and emulsification techniques24 have already been studied. They showed that chitosan in solution enables the transport of even macro drug molecules across the nasal membrane. Moreover, application of chitosan in microspheric form enhances transfusion of the macromolecules. On the other hand, PVA, a hydrophilic biocompatible polymeric excipient which is being widely used in biomedical and pharmaceutical applications. The polymer has unique gelling characteristics, which in turn makes it responsible for its adhesiveness and film-formation properties. PVA is used in the present study to investigate the effect of polymeric blend along with chitosan to explore the possibility for the improvement of mucoadhesive and drug release properties of insulin from the system either of which to be used alone or in combination with each other in appropriate ratios to understand if they could synergic mucoadhesiveness and drug release patterns. When PVA comes into contact with the physiological fluid, it increases the viscosity and forms thin hydrogel film like structure on nasal surface.
Mucoadhesive polyvinyl alcohol hydrogels produced by freezing/thawing processes: applications in the development of wound healing systems. But the rate, degree and extent of absorption from PVA formed hydrogels needed to be studied more in detail which will be discussed in this paper further. It has been reported that such chitosan/PVA blend, forms in situ gel once it comes in contact with nasal muciliary membrane and minimizes the nasal mucociliary clearance of the formulation. The blend has also been used by Minoura et al. (1998) & Koyanoa et al. (2000) to study the surface properties and their relationship with the cell attachment or growth behaviour. Kim et al. (2002) has studied electrical charge sensitivity, Wang et al. (2004) for pH sensitivity and by Tang et al. (2007) for rheological characterization of the blend.25
The key point which has to be clearly emphasized is that very rarely feasibility of large scale production of industrial batches of microspheric sprayed dried micromolecules including insulin have been studies. Therefore one of our concerns in this paper was the industrial scale up of the optimized formulation with spray drying technique more in detail. To understand the possible obstacles ahead of large scale production, the experiments have been performed in a small laboratory scales primarily before scaling it up.
Therefore, the purpose of the present study was to design an fabricate an appropriate and suitable applicable polymeric microspheres for intranasal delivery of insulin using a novel optimized formulation based on industrial level spray drying technique and to realize the possible barriers in scale up process of its large scale production, considering the effectiveness of polyvinyl alcohol (PVA) and chitosan to increase mucoadhesivness, gelling ability and ultimately effective release behavior pattern of insulin. It is worthy to state that spray dried technique has been studied in accordance of being a well-defined means for industrialization. Moreover, it was estimated that Spray dried insulin particles would retain biological activity of slow in-vitro assay/ The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation.26
The designed and produced microspheres were further characterized and intranasal absorption of insulin encapsulated in the polymeric matrix was evaluated in vitro and in vivo after intranasal administration.
Materials
Dry powder recombinant human insulin, medium molecular weight chitosan (200-800 cP) and polyvinyl alcohol (PVA) 40kDa Mw were all obtained from Sigma-Aldrich (USA). Recombinant Regular Human Insulin 100 I.U was purchased from Exir (Iran), monobasic potassium phosphate, glacial acetic acid and acetonitrile were also purchased from Merck (Darmstadt, Germany). Rest of the chemicals which have been used in this study were of high analytical grade.
Methods
Preparation of standard solution: Concentrated solution of insulin was prepared by dissolving 30 mg of insulin dry powder in 5mL 0.1 mole/L HCl acid, 25mL of distilled water was added to the acidic solution and was mixed further in a mechanical stirrer. The final stock solution was diluted appropriately to prepare standard dilutions of different concentrations ranging from 0.5, 0.25, 0.125, 0.0625, 0.0312 and 0.015 mg/mL.
Preparation of the microspheres: Microspheres were prepared using spray drying technique for solutions containing insulin, chitosan and PVA at different ratios in 1% acetic acid as a solvent. The solutions were agitated for 10 minutes with a magnetic stirrer (Heidolph-D-91126, Germany) at speed of 1300rpm. Different composition of the prepared formulations is shown in Table 1. The concentration of insulin was kept constant by addition the fixed amount (6mL) of Recombinant Human Insulin 100 IU solution in all the formulations i.e., each 1 IU is equivalent to 0.0347 mg of human insulin. Chitosan based microspheres were prepared by dissolving 100mg chitosan in 100mL of 1% v/v acetic acid which were well stirred for 10min. Different concentrations of 0.5% and 0.25% (w/v) were formed from the already prepared fresh solution by stirring at 1300 rpm. Fixed amount of Recombinant Human Insulin (6mL) was added further drop wise using insulin injection syringe to the solutions while stirring on an agitator. PVA polymeric solutions were also prepared with the same method as described above. For this, 100mL distilled water was heated up to about 70-75˚C and the temperature was kept constant. 100 mg of PVA powder was added slowly to prevent the formation of agglomerates. It was then well stirred by the same speed to avoid the possible agglomeration of the polymer. Then solution was further stirred for another 10 min to obtain clear solution. Different compositions of the chitosan/PVA polymeric blend were prepared according to the ratios indicated in Table 2. The solutions were stirred at 1300 rpm for 10 min and constant amount of Regular Human Recombinant Insulin 100 I.U. was added drop by drop using a syringe. The solutions were then dried using spray drying technique to obtained desired spherical microspheres of suitable particle size. For this, the spray dryer (Buchi 191, Switzerland), was set to have the inlet temperature of 100°C and outlet temperature of 90°C. Aspirator was 80% and pump setting was 8mL/min. The obtained microspheres were collected and maintained in a desiccator for future analysis.
Group A, Containing 100 mg Chitosan in 1% Acetic acid (w/v%) |
||
Formulation |
Chitosan Amount |
Composition (%) |
A1 |
1000 mg |
1% |
A2 |
0.50% |
|
A3 |
0.25% |
|
Group B, Containing Different Ratios of Chitosan: PVA |
||
Formulation |
Chitosan: PVA |
Amount of Chitosan: PVA (mg) |
B1 |
50:50:00 |
500: 500 |
B2 |
70:30:00 |
700: 300 |
Group C, Containing only PVA (1%) |
||
Formulation |
PVA Amount |
PVA Composition (w/v % in Distilled water) |
C |
1000 mg |
1% |
Table 1 Compositions of various formulations based on different concentrations of chitosan and polyvinyl alcohol.
CV % |
Mean |
Measured Concentration (µg/mL) |
Real Concentration (µg/mL) |
||
Day3 |
Day2 |
Day1 |
|||
0.78 |
0.508 |
0.509 |
0.503 |
0.512 |
0.5 |
2.17 |
0.253 |
0.252 |
0. 248 |
0.259 |
0.25 |
2.43 |
0.123 |
0.127 |
0.123 |
0.121 |
0.125 |
3.23 |
0.065 |
0.0677 |
0.064 |
0.064 |
0.0625 |
Table 2 Inter day variation of the method of analysis.
Measurement of encapsulation efficacy
Encapsulation efficacy was determined by dispersing 10mg of each microspheres in 2.5mL methanol: acetic acid (1:1) ratio. The obtained suspensions were kept on a rotary extractor overnight. The supernatant layer was taken out after 12h without being centrifuged. It was further diluted with HPLC mobile phase composed of 45% acetonitrile and 55% buffer solution pH 7.5 and was injected into the HPLC instrument for the analysis.
Scanning Electron Microscopy
Morphology of each sample was studied by Scanning Electron Microscopy (SEM) (Philips XL 30 scanning microscope, Philips, The Netherlands) at 25KeV. The samples were placed onto an aluminum stub using double sided adhesive carbon disc (SCD005 Sputter coater, Bal-Tec, DE). The samples were sputter coated with gold 20mA for 4 minute prior to analysis.
HPLC Chromatography
Several methods such as capillary electrophoresis and gradient HPLC systems have been evaluated in order to overcome the need for gradient HPLC system for analysis of insulin.27,28 It is necessary to apply two or three pumps in gradient HPLC method. As stated before the mobile phase of the systems was composed of 45% acetonitrile and 55% buffer solution pH 7.5. The buffer solution was prepared fresh at the time of analysis by mixing 0.01M monobasic potassium phosphate and KOH 8N solutions. The KOH 8 N solution was used for adjusting the buffer pH at 7.5. The analysis was carried out at a flow rate of 1mL/min with 1100 psi pressure. The instrument (HPLC-Cecil-CE 4104, England) was equipped to UV detector (Cecil-CE 4300, England) and the measurement was carried out at 210 nm with ODS-3.5µm 125×4 mm column (Perfectsil Target, Germany). Phosphate buffer pH 7.5 was produced by dissolving 6.8 g potassium dihydrogen phosphate in 1 liter of distilled water and the desired pH was adjusted to 7.5 using 8N potassium hydroxide. The buffer solution was then filtered with syringe filter and was mixed with acetonitrile in ratio of 45:55. 4.5 mg of standard insulin powder was dissolved in 9mL of deionized distilled water to obtain the stock solution having concentration of 0.5 mg/mL. Different concentrations of 0.25, 0.125 and 0.0625 mg/mL were further prepared from the stock solution. 50µL of each sample was then injected into the HPLC system using the aforementioned mobile phase at 210nm. The tests were repeated in triplicate.
125mL of 0.2M potassium dihydrogen phosphate was mixed with 14mL of 0.2M sodium hydroxide. The mixture was then volume up to 500mL with distilled water to obtain buffer system solution having pH 6.5 considered as a pH of the nasal mucosa. 30 mg of each formulation was thoroughly weighted and were transferred to different labeled vials. 2mL of the previously fresh prepared buffer solution was added to each vial. The vials were kept on a mechanical shaker and the sampling was performed at different time intervals of 0.16, 0.33, 0.5, 0.66, 0.83, 1, 2, 3, 4, 5, 12 and 24 h. Within each of the specific time periods, 0.5mL of the solutions was taken out and immediately were replaced with the same fresh buffer system. Samples were then centrifuged at 10000rpm for 10min. 50µL of supernatant was injected into HPLC instrument equipped with UV visible detector having 125x4 mm column in flow rate of 1mL/min and particles size of 3.5µm. Composition of the mobile phase system was as described above and measurement was carried out at 210nm. Amount of insulin released was measured based on the obtained AUC of insulin chromatogram and the previously constructed calibration curve. The concentration and the amount of the drug released in various time intervals were calculated and ultimately cumulative percentage of drug release was plotted.
Measurement of insulin release efficacy
Trapezoidal method was used to calculate the release efficacy of insulin from each formulation. Release efficacy percentage was calculated using the following formula:
Where AUC is the area under curve of drug release chromatogram and AUCs is the area under curve of standard sample.
Measurement of Mean Dissolution Time (MDT)
To measure mean dissolution time (MDT), trapezoidal method was used again by dividing the area under curve of the release diagram by final release percentage of each particular formulation.
24 female Wistar rats weighting from 200-250 g were obtained from the animal house of the faculty of pharmacy, Zabol University of Medical Science following the code of practice for the established university animal ethics. The Helsinki animal ethics law for evaluation of the absorption mechanism of insulin from the nasal mucosa was also considered.
All in vivo experiments were carried out according to the National Institute of Health guidelines for the care and use of laboratory animals and to the European Community Council Directive for the care and use of laboratory animals of 24 November 1986 (86/609/ EEC) and were approved by the local ethics committee.
The animals were kept in light and dark conditions for 24h, at mean temperature of 20-25˚C with relative humidity of 45-65%. They were further maintained fasted for 12 before initiation of the study having free access to water. The animals were classified into 4 different groups each containing 6 rats, anaesthetized in a saturated chloroform chamber 2h before instillation of the formulations and were controlled every 1 h to make sure that the animals are still alive until the scheduled time is over. The control group (A), was composed of the animals that were administered 0.5mL phosphate buffer pH 7.4 via nasal route. Standard group (B), received fixed amount of insulin equivalent to 2mg of the Regular Human Insulin intranasally. 17mg of the two optimized formulations (A3 and B3) were suspended in 2mL phosphate buffer pH 7.4 and were then instilled using 2mL syringe to the nasal cavities of the rats in groups C and D respectively. 0.5mL of blood samples were taken out from the tail veins of the animals 2h before initiation of the study and consequently at the time intervals of 0, 1.5, 3, 4.5, 6, 7.5 and 9h after instillation of the optimized formulations. Blood samples were centrifuged at 10000rpm for 10 min to obtain clear and supernatant solution. The solutions were then filtered with 0.45µm syringe filter and the concentration of the glucose in the blood samples was measured using HPLC method after injection of appropriate dilutions i.e., 100µl.
All the studies were performed in triplicate and data are presented as mean ± SD (standard deviation). Statistical significance was analyzed using Student’s t-test and one way ANOVA method. The p value <0.05 was considered as significant.
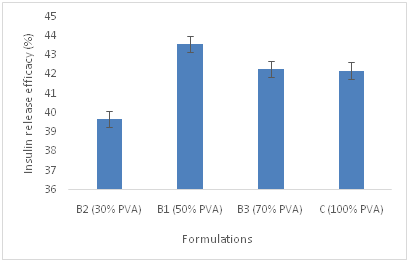
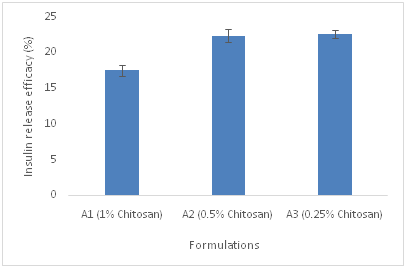
The loading percentage and release efficacy of insulin in three different groups of polymeric microspheres A, B and C has been shown in Table 3. Cumulative release profile of insulin from microspheres id different formulations was also depicted in Figure 1. Based on drug release profile and MDT data from Table 3, presence of PVA in the polymeric matrix of the microspheres increases the release rate of insulin from encapsulated polymeric matrix which is not significant (p<0.05). Insulin was completely released from formulations of group B i.e., B1, B2 and B3 containing PVA and chitosan in different proportions and C group in which only PVA has been used during the first 15h of the study. In contrast, only 35-50% of Insulin was released from A1, A2, A3 chitosan based microspheres for the same duration of time. The variance in release pattern may be attributed to the difference in the solubility of PVA and chitosan as the polymeric vehicles in the buffer solution pH 6.5. PVA is more water soluble than Chitosan in pH 6.5 and its solubility depends on degree of polymerization and percentage of hydrolysis. For this reason the release rate of insulin from PVA microspheres is impressive. All the formulations showed burst release pattern from time 0 to 1h. Increasing the percentage of chitosan in formulations of group A, decreases the release rate of insulin from the microspheres which is not significant (p<0.05). The overall cumulative release of insulin from various group of formulations are shown in Figure 1. The physicochemical characteristics of the microspheres such as particle size, drug loading, percentage yield, MDT and recovery percentage were summarized also in Table 3. According to Table 3, decreasing the concentration of chitosan in group B formulation containing varying ratios of chitosan and PVA, decreases the particle size of the group B microspheres further (p<0.05). The size distribution of the microspheres differs. Application of 50% PVA in the polymeric blend leads to formation of microspheres with smaller mean particle size compared to PVA only containing polymeric microspheres. According to Figure 2a and 2b, it is also evident that decreasing the concentration of chitosan in A and B groups of formulations, decreases the MDT magnitude, suggesting that the dissolution of group B which contains blend of chitosan and PVA is faster than group A containing chitosan alone. Among B group of formulations, B3 had the least MDT value which would be supported by the fact of prolonging drug release from the microspheres due to the desirable adhesion to the nasal mucosa. Presence of PVA in the structure of the microspheres, decreases the loading percentage of insulin from the vehicle (p<0.05). To better understand the morphology of the microspheres, SEM images of the selected formulations was presented in Figure 3. According to Figure 3, microspheres are spherical in shape, almost densely packed to each other. It is more evident in group B and C formulations, where PVA is present, more spherical microspheres have obtained. In chitosan only based microspheres, i.e., group A, decreasing the concentration of chitosan leads to the formation of more spherical shaped particles which are loosely packed. The formation of the spherical particles with the smooth surface increased by augmentation of chitosan concentration in deep particles structure. In group B formulations, increasing the ratio of chitosan in the polymeric blend increases the smoothness of the microspheres surface.
Formulations |
Mean Particle Size (µm) (mean± SD) |
Loading %(mean± SD) |
Yield % |
k (mean± SD) |
Release Efficacy % (mean± SD) |
Mean Dissolution Time (h) (mean± SD) |
A1 |
1.116±0.507 |
4.3±02 |
27.1±04 |
0.148±0.009 |
17.110±0.963 |
41.168±1.860 |
A2 |
2.083±0.664 |
4.3±03 |
27.1±02 |
0.165±0.007 |
20.803±0.963 |
34.461±1.104 |
A3 |
1.666±0.752 |
4.3±02 |
27.18±02 |
0.165±0.014 |
21.290±0.963 |
32.655±3.316 |
B1 |
1.166±0.408 |
2.6±01 |
25.31±03 |
1.618±1.997 |
43.693±0.963 |
13.750±1.838 |
B2 |
1.750±0.612 |
2.6±02 |
19.7±02 |
0.306±0.203 |
39.893±0.963 |
14.740±1.335 |
B3 |
1.50±0.547 |
2.6±01 |
12.65±01 |
0.318±0.096 |
42.639±0.963 |
13.766±0.300 |
C |
1.61±0.435 |
2.2±02 |
15.72±03 |
0.578±0.506 |
43.080±0.963 |
14.621±2.81 |
Table 3 Physical characteristic, release rate constant (k), mean particles size, percentage of recovery in spray dryer, drug loading percentage, release efficacy percentage and mean dissolution time of groups A, B and C formulations.
The blood plasma profiles of glucose concentration after intranasal administration of the A3 and B3 formulations are shown in Figure 4. It is well understood that, increasing the concentration of chitosan reduces the extent of insulin absorption via the intranasal rout in groups or rats. It would be justified by the fact that mucoadhesiveness and tight junction opening characteristics of chitosan molecules is responsible for the same phenomenon. These inherent properties of chitosan as a natural polymer have been reported previously.6 Polycationic structure and the positive surface charge of chitosan as a natural polymer could be the key element for this issue which has to be considered in large scale production of the formulation.
Based on this study, it was confirmed that the optimized composition of polymeric blend as well as manufacturing techniques used in the production of the microspheres in both small and large industrial levels are effective and determining factors on in vitro insulin release pattern and concerned physicochemical characteristics of the particles including rate and extent of drug release from the microspheres. These parameters have significant effect on in vivo behavior of the optimized formulations that directly affect the rate and the extent of absorption of insulin as a macromolecule drug moiety via intranasal rout of administration.
None.
The author declares no conflict of interest.

©2017 Zadeh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.