MOJ
eISSN: 2573-2951


Mini Review Volume 3 Issue 2
Department of Pharmaceutics, KB Institute of Pharmaceutical Education & Research, India
Correspondence: Divyesh H Shastri, Assistant Professor, KB Institute of Pharmaceutical Education & Research, Gh-6 Corner, Sector-23, Kadi Sarva Vishwavidyalaya, Gandhinagar, Gujarat, India, Tel +919426420259
Received: October 29, 2016 | Published: March 14, 2017
Citation: Shastri DH. Thiolated chitosan: a boon to ocular delivery of therapeutics. MOJ Bioequiv Availab. 2017;3(2):34-37. DOI: 10.15406/mojbb.2017.03.00029
Despite numerous scientific efforts many challenges need to be overcome in the ocular delivery because eyes have special and complex anatomic structures. The main problem with the conventional dosage forms is poor ocular bioavailability due to minimum ocular residence time, because of various anatomical and pathophysiological barriers prevailing in the eye. For this reason, much effort has been put into developing nanotechnology-based drug delivery systems to improve ocular bioavailability of drugs by increasing the precorneal residence time. Polymers with thiol groups provide much higher adhesive properties than polymers generally considered to be mucoadhesive. Chitosan and its derivatives are useful polymeric biomaterials that have found a number of applications in drug delivery especially via ocular route. Thiolated derivatives of chitosan known as thiomers produced via immobilization of thiol groups on the primary amino groups of chitosan backbone has improved mucoadhesive properties, permeation enhancement and anti-protease activity. Moreover, it is biocompatible, biodegradable and non-toxic and has mucoadhesion properties by establishment of electrostatic interactions with mucin. Many drugs and therapeutics can be administered via nanoparticulate form or as an in situ gel forming systems through the ocular route using thiolated chitosan. This mini review provides an insight into the various approaches using thiolated chitosan as nanocarriers for ocular drug delivery, summarizes recent findings and its applications in the field of ocular drug delivery.
Keywords: thiolated chitosan, mucoadhesive, penetration enhancer, in-situ gel, nanoparticulate, ophthalmic drug delivery, film
TCS, thiolated chitosan; CS, chitosan; CS-TBA, cs-4-thio-butyl-amdine; PACA, poly alkyl cyanoacrylate; PLGA, poly lactic-co-glycolic acid; PCL, poly epsilon-caprolactone; HCE, human corneal epithelial cell.
Ocular drug delivery systems provide local as well as systemic delivery of the drugs. Decrease in patient compliance and high tear turnover rate, loss of therapeutics through nasolacrimal drainage resulting in 10 to 12 fold decrease in the bioavailability of the drugs when administered through Conventional formulations used today for many ophthalmic disorders and infections like bacterial Keratoconjunctivitis failed many times. The anatomy-physiology of the ocular tissue leads to poor absorption of drugs over the corneal surface, ultimately results in short duration of action.1 Because the cornea is a membrane-barrier containing both hydrophilic and lipophilic layers, drug substances having both hydrophilic and lipophilic characteristics permeate it most effectively thus, the ocular bioavailability of the drugs can be significantly improved by increasing the precorneal residence time. Several conventional approaches such as inserts,2 collagen shields,3 and colloidal systems, such as liposomes,4,5 nanoparticles6,7 and nanocapsules8,9 have been tried for the treatment of ocular disorders with a view to prolong the precorneal drug residence time and thereby reducing the drug elimination.10 With the use of nanotechnology based ocular drug delivery i.e., solid-lipid nanoparticles, nanosuspensions, niosomes and dendrimers have solved many issues related to poorly soluble drugs like, dexamethasone, ganciclovir etc.11 Moreover, these novel dosage forms have a few demerits also like poor patient compliance. Out of these novel formulations; in-situ gel and nanoparticles have been established as the promising one in the field of novel advanced delivery systems which can offer more protective and effective means of therapy for nearly inaccessible diseases or syndromes of eyes.
Thiolated Chitosan (TCS) based dosage forms are gaining popularity due to high mucoadhesive strength and prolonged drug release properties. The derivatives of primary amino groups of chitosan (CS) with thiol groups results into the formation of TCS.12 Various mucoadhesive excipients (natural/synthetic) have been developed and their mucoadhesive properties can be explained with the help of interaction of glycoproteins of the mucous through non-covalent bonds i.e., hydrogen bonds, ionic bonds and week vander-waals forces.13,14
Chitosan (CS), a cationic polysaccharide, has been widely used in ophthalmic therapy1 obtained naturally by alkali deacetylation of chitin and various industrial sources i.e. lobster and crab, shell wastes of shrimp etc.15,16 The primary amino groups of chitosan can be easily modified chemically i.e., salt formation with acids. CS is insoluble in alkaline and neutral pH17,18 but the amino group can be easily protonated at acidic pH leading to increase in solubility. Moreover, favorable enzymatic biodegradability, non-toxicity and biocompatibility CS leads to a considerable attention by the scientists for its favorable use as a novel excipients for the drug delivery systems and thus, since 2002 it was included in European Pharmacopoeia.19 Furthermore, CS has penetration-enhancing properties and thus, has attracted significant attention as being a potential absorption enhancer for transport across the mucosal epithelia. Because of poor aqueous solubility of CS at pH above 6 limits its effectiveness at the site of drug absorption. Above pH 6, CS loses its positive-charge density, and induces the formation of aggregates and precipitates.20 This problem can be overcome by the use of thiolated chitosan (TCS), comprising a new generation of mucoadhesive polymers-thiolated polymers or thiomers.21
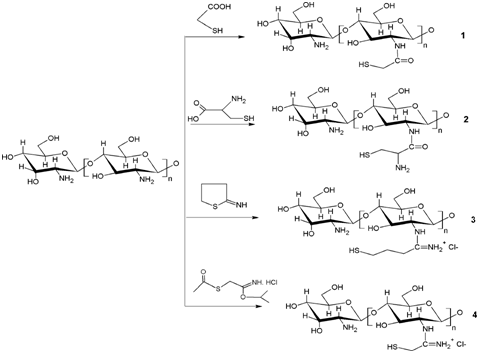
Till date there are three different types of thiolated chitosan derivatives have been synthesized: CS-thioglycolic acid conjugates, CS-Cysteine conjugates and CS-4-thio-butyl-amdine (CS-TBA) conjugates (Figure 1).22,23 The larger the amount of immobilized thiol groups the greater will be the improvement in mucoadhesive properties.21 TCS have numbers of characteristic features over CS i.e., significant mucoadhesive strength, excellent permeation enhancer and strong cohesive properties make them a first choice for sustained release preparations.23-25 The combined formulations i.e., nanoparticulate in situ gel have their own advantages for example, in-situ gel remains on the ocular surface for 12-15 hr, whereas thiolated chitosan (TCS) nanoparticles are mucoadhesive, so can retain the drug moiety over the corneal surface for prolong period of time.
In Situ Gelling Drug Delivery
Thiolated chitosans display in situ gelling properties due to the oxidation of thiol groups at physiological pH-values, which results in the formation of inter and intramolecular disulfide bonds. This cross-linking process can be observed within the pH range of 5-6.8. The in situ gelling behavior of thiolated chitosans was characterized in vitro by rheological measurements. The sol-gel transition of thiolated chitosans at pH 5.5 was completed after 2 hours when highly cross-linked gels were formed. In parallel, a significant decrease in the thiol group content of the polymers was observed, indicating the formation of disulfide bonds.26,27 The rheological properties of plain chitosan remained constant indicating a clear correlation between the total amount of polymer-linked thiol groups and the increase in elasticity of the gel. The more the thiol groups immobilized on chitosan, the higher was the elastic modulus of thiolated chitosan 26,27 which can also be explained by the formation of covalent disulfide bonds between the thiol groups and the mucus glycoproteins, which are much stronger than non-covalent bonds. Chitosan based in situ gelling drug delivery system using polyacrylic acid evaluated and found to be in sol form at pH 6.0±0.1 and formed gel at physiological pH 7.4±0.1. An aqueous 1%(m/v) solution of Chitosan-thioglycolic acid conjugate (TCS) can improve the in situ gelling characteristics of chitosan even further upto 16500 fold when applied to the mucosal surface in a liquid form. The strong increase in viscosity attributed to a cross linking taken place with the mucosal surface.28
Nanoparticulate drug delivery
Nanocarriers such as nanoparticles have wide applications in ophthalmology as they have capacity to deliver ocular drugs to specific target sites. Different polymers are used in nanoparticle formulations. Nanoparticles are prepared from different biodegradable polymers like poly (lactic acid). poly (alkyl cyanoacrylate) (PACA), poly(lactic-co-glycolic acid)(PLGA), poly(epsilon-caprolactone) (PCL), as well as different natural polymers like chitosan, gelatin, sodium alginate, and albumin, can be used effectively for drug delivery to ocular tissues. Nanosuspensions can be valuable for the drugs which have poor solubility in lachrymal fluids. The nanoparticulate nature of the drug shows sustained release effect by increasing its residence time in the cul-de-sac. The nanoparticles protect the drug against agents which cause degradation.29
Chitosan based nanoparticles disintegrate very rapidly unless they are combined with multivalent anionic compounds i.e., sodium sulfate29 or alginate resulting in stabilization of the system by an ionic cross-linking process. Addition of multivalent anionic compounds mentioned above reduces the mucoadhesive properties of the chitosan very strongly. While thiolated chitosan based nanoparticles do not disintegrate because of the formation of disulfide bonds within the polymeric network stabilizing the microparticles strongly and providing controlled drug release. Due to immobilization of thiol groups on chitosan, addition of multivalent anionic compounds to the thiolated chitosan based nanoparticles leads to improve the mucoadhesion properties very strongly in contrast to chitosan.30
Ocular drug delivery based on thiolated chitosan-sodium alginate NPs (TCS-SA NPs) from a modified thiolated chitosan with a high degree of thiol substitution of TCS, up to 1,411.01±4.02 μmol/g showed higher mucoadhesive properties, good in vitro cytocompatibility, greater amounts of drug delivery into HCE cells in vitro and cornea in vivo showing good potential for ocular drug delivery [31,32]. Large polyanionic molecules i.e., DNA based drug and siRNA a stable drug complex can be formed with chitosan with sufficiently high ratio of polycationic molecule to polymer. Due to small particle size (<100nm) and the positive charge developed on the nanoparticles, endocytosis can be achieved very quickly and thereby chitosan DNA based drug complex can be delivered into the body very easily with increased bioavailability without affecting the stability of the DNA based drugs in the complex [33-36]. Modifying the chitosan with the thiol group i.e., chitosan/plasmid nanoparticles can improve the transfection efficiency of the protected plasmid forming intra chain disulphide bonds within the complex.37 Self-branching of chitosan can improve the gene transfer properties i.e. two and five times higher than those of Lipofectamine and Exgen, respectively.38 The transfection rate of thiolated chitosan/plasmid nanoparticles was observed five times higher than unmodified chitosan/pDNA nanoparticles. This property could be further improved by trimethylation of the remaining primary amino groups and thereby raising the cationic character of thiolated chitosan.39 Chitosan-DNA NPs has the proper nanoparticle size and positive zeta potential charge and can become a first choice for corneal gene therapy. Corneal fibroblasts can express the transgene green fluorescent protein.40
Ocular drug delivery of nanoparticulate prulifloxacin in situ gel based on thiolated chitosan with mean particle size 16nm and 80% drug entrapment efficiency showed gelation pH near pH 7.2±0.2, found excellent mucoadhesion properties, non-irritant and exhibiting sustained drug release over the cornea.
Thus it can be concluded that thiolated chitosan derivatives seem to be promising new excipients, which should stabilize themselves once applied on the site of drug delivery. Moreover, the thiolated chitosan offers a promising alternative to be used as nanomedicine for improved transfection efficiency. Thus, the review article discussed the combined advantages of both these approaches thiolated chitosan nanoparticles and in-situ gel with a view to increase the drug residence time of the nanoparticles to the corneal surface and thereby increasing the ocular bioavailability. Thus, there is a good scope for delivery of therapeutics via ocular route with the help of thiolated chitosan. Special properties of this nanoparticulate in situ gel technology using thiolated chitosan can be considered as a new beginning in formulation technologies in the imminent years.
Besides teaching, author is also involved in research activities on modified polymers at KSV University.
The author declares no conflict of interest.

©2017 Shastri. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.