MOJ
eISSN: 2573-2935


Brief Report Volume 7 Issue 1
1Program Director, TeDDs on Chapel, Canada
2Executive Director, TeDDs on Chapel, Canada
Correspondence: Christopher Ashton, BEng, MD, MBA (Finance), Program Director, TeDDs on Chapel, Canada, Tel +4169996805
Received: August 26, 2022 | Published: October 3, 2022
Citation: Ashton C, Duffie D. Crossed wires: the hall effect in substance use disorder. MOJ Addict Med Ther. 2022;7(1):1-2. DOI: 10.15406/mojamt.2022.07.00150
The underlying neuroscience of substance use disorder is becoming well elaborated. Nonetheless, some of the more subtle symptomatology is not well matched with underlying organic processes identified to date. The ability to explain mental phenomena with underlying brain processes is a strong part of the literature and valuable to those caring for persons. This article draws on current knowledge of the fundamentals of substance use disorder and expands on current literature surrounding axonal demyelination to suggest a likely mechanism for thought disorders commonly experienced by persons in recovery. Viewing demyelination and conduction through an analogue lens is more likely to represent the physics involved more accurately than an ‘on or off’ signalling model as associated with action potentials. Additionally, this approach is thought to better enunciate the underlying physiology behind the mental features characteristic to the disorder.
I recently attended a meeting of Alcoholics Anonymous, where a very distinguished appearing man expressed his need to attend a meeting to be rid of the 'pollution going on upstairs,' motioning to his head. His statement needed no further clarification as the majority of members identified with his condition, also called 'noise' and similar descriptions.
Clinically, personally and empirically, I have observed and experienced this 'pollution' phenomenon very frequently among members of the recovery community. It is most apparent in persons in the first few months of abstinence from substances and is simply referred to as 'the disease' in recovery circles. It is certainly hallmark for persons with substance use disorder (SUD), refers to a mental phenomenon whereby persons' thought patterns become uncontrollably chaotic and is frequently anxiety and dysphoria promoting. It also seems to improve with length of time spent in recovery yet remains somewhat persistent and stimulated by stress.
An established description for this involuntary transient thought disorder is absent from the DSM V criteria for SUD yet is a common source of discomfort and morbidity for those in recovery. For the purposes of this paper, it will be referred to as chaotic thinking (former drinkers will have no problem relating to this). This paper's objective is to utilize the current state of neurobiological research and offer a novel perspective, building on what is known of the effect of substances on neuronal activity in specific areas and pathways in the limbic system.
Continued and newly emerging findings from neurobehavioral research have clearly established substance use disorder as having an organic basis. Consistent patterns of dysfunctional neuroadaptations in gray matter structures and their connections (white matter) among all persons who suffer (ed) from SUD are apparent and attributed to genetics and environmental influences. Nonetheless, application of this knowledge by clinicians and treatment specialists is very much lacking.1 More than simply the inability to stay current with the literature, understanding the impact these maladaptations manifest within the complex thinking of persons in active addiction and recovery is challenging.
Research findings clearly indicate cognitive impairments in persons in early recovery from substances (references). Much of the literature articulates prefrontal cortex (PFC) dysfunction as largely implicated in the impaired response inhibition and salience attribution (iRISA) framework.2 In terms of a fundamental framework regarding mechanisms surrounding addiction and relapse, the iRISA model is excellent. However, when one searches for a scientific explanation for some of the more subtle mental symptoms of SUD among persons in recovery, iRISA falls short.
Current studies implicate dysfunction of the PFC gray matter (cell bodies) with impaired aggregate inhibitory response, impaired reward and risk encoding along with decreased axonal activity (white matter) due to demyelination as fundamental to relapse risk. The majority axonal projections involved belong to the mesocortical pathway (PFC projections primarily to nucleus accumbens) and the highly complex medial forebrain bundle (PFC projections to multiple areas of the limbic system, including the amygdala, ventral tegmental area hippocampus and hypothalamus). In combination with a down regulated 'reward system,' PFC dysfunction is thought to lack adequate inhibitory strength to counteract cues and cravings arising in the deeper limbic structures. Exaggerated by incremental stresses superimposed on a typical high allostatis load, excessive catecholamines, cortisol and corticotropin releasing factor (CRF) both make inhibitory control worse as well as impair recovery to more neurotypical function.
This seems a logical conclusion, supported by imaging data through fMRI on this issue.3 However, while it may be adequate to explain relapse risk and phenomena, iRISA's scope does not adequately explain the more subtle cognitive and emotional dysfunctions responsible and recognized as 'the disease' within recovery members. Exploration of the basic science of the neuron through a different lens gives a greater explanation (acknowledging that this is theoretical) of the real-life experiences of the person in recovery from SUD. Much of current thinking surrounding functionality of the neuron is based on action potentials as existing/transmitting or not, along myelinated axons across a chemical synapse (akin to a digital process). Aggregate inhibitory vs excitatory signalling strength among the various components of the limbic system determine and maintain homeostasis (Figure 1 & 2). We portend that it may be more accurate to describe resultant action potentials occurring at high enough frequency and varying amplitudes that signal transmission be viewed through the lens of analogue systems. Additionally, with respect to demyelination of axons due to substances, we also suggest that this process be more accurately be described as varying degrees of demyelination, with efficient and partially efficient signal transmission.
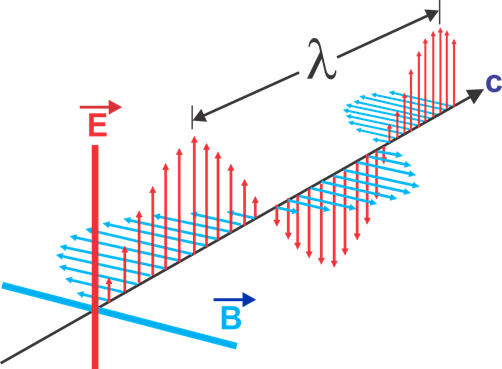
Figure 2 The electromagnetic wave where B is magnetic field; C represents current, E represents electric field, lambda represents wavelength.
Within large tracts of white matter, there would be variably myelinated from fully (efficient) to partial (inefficient) and to non-myelinated axons (non-conducting). What would this then imply in the context of analogue systems and electrical current instead of action potentials describing energy passed along neurons? The myelin sheath is the protective, fatty coating surrounding axons, similar to the protective insulation around electrical wires. This coating enables the electrical impulses between neurons to conduct efficiently, minimizing energy loss due to potential cross talk among neurons. Full myelination of axons occurs over a lengthy time period, with those in the prefrontal cortex being the last to fully myelinate at approximately age 25 years.
Several drugs and their adulterants can cause demyelination by a chemical-induced neurotoxicity mechanism or immune system deregulation, both in developmental periods and adulthood.4,5 It is postulated that among these, cocaine and crystal methamphetamine have a more rapid and neurotoxic effect, given their ability to cross the blood-brain barrier. With varying degrees of myelination, one could expect considerable inefficient conduction in the larger, more discrete pathways such as the mesocortical. Viewing the 'action potential' more accurately as an electromagnetic wave, one sees propagation of current along the axonal axis with a corresponding perpendicular magnetic wave.
These waveforms, of course, confined to the axon through its insulating myelin sheath. However, given degrees of demyelination, one can anticipate energy dissipation, especially of the magnetic field. This field in turn affects current and voltage amplitudes of neighbouring neurons through a Hall effect.6 The resultant aberrations in information transfer along an axonal bundle would be significant and relatively unpredictable. Higher physiologic stress states would potentiate this effect through catecholamine enhancement of signal strength.
This Hall effect has the potential to account for the phenomenon of chaotic thinking, experienced by persons at all phases of recovery from SUD. While there is no literature on the approach to explain and treat this aspect of 'the disease,' further exploration of this concept has merit in assisting persons with SUD understand the thought disorders implicit. As an example possibility, research to measure the Hall Coefficient in larger white matter pathways in lab animals could offer a quantitative approach to understanding efficiency of conductivity in normal axons. Through remeasurement of the coefficient for animals using systematic protocols for the addition of addictive substances, would indicate degree of injury to myelin sheaths related to various substances and administration protocols. The need for measures to enhance remyelination for persons in recovery is made even further important given the greater potential for morbidity on this basis.
None.
The authors declare that there is no conflict of interest.

©2022 Ashton, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.