Journal of
eISSN: 2574-8114


Research Article Volume 9 Issue 6
School of Textile Science and Engineering, Tiangong University, Tianjin, China
Correspondence: Anik Das, School of Textile Science and Engineering, Tiangong University, Tianjin, China
Received: December 02, 2023 | Published: December 14, 2023
Citation: Anik D, Xu L. Synthesis and investigation of dyeing properties of 8-hydroxyquinoline based azo dyes & 1-naphthylamine based azo dyes. J Textile Eng Fashion Technol. 2023;9(6):182-188. DOI: 10.15406/jteft.2023.09.00354
A facile and well-known synthetic strategy has been used to prepare this novel 8-hydroxyquinoline-based azo dye to apply as a disperse dye to polyester fabric. In this method, 8-hydroxyquinoline possessing a “privileged structure” which is made up of two rings, a phenol ring fused with pyridine ring, has been coupled with diazonium salt obtained from 1-Napthylamine. As-synthesized dyes are used as disperse to polyester fabric, and the dyed samples were characterized for color fastness to washing, rubbing, and perspiration. The fastness testing results indicate that the dyes have a good affinity to PET fabric as the dyed PET fabrics have moderate to excellent fastness to washing and perspiration and good fastness to rubbing. The dyeing property of 8-hydroxyquinoline-based dyes is very promising and can be considered as the potential candidate to meet the actual demand of PET fabric dyeing in the textile industry.
Keywords: textiles, fibers, azo dyes, fabric
Textiles are colored by applying various dyes and their shades at any stage of production, from fibers to finished garments. There are natural dyes, extracted from plants, animals, or minerals, and synthetic dyes, created in labs with chemicals, sometimes containing metals. Azo dyes are popular for polyester because of their brilliant colors, wide range of shades, excellent fastness, and affordability. These dyes are synthetic, containing an azo bond (-N=N-) derived from aromatic amines, nitro, and nitroso compounds. While 8-hydroxyquinoline-based azo dyes are less studied for polyester, they offer potential for reducing environmental impact and improving washing and rubbing fastness. 8-hydroxyquinoline, a derivative of quinoline, is a strong metal chelator with diverse applications, including antimicrobial, antioxidant, and anticancer properties. It can also be used as an insecticide, antifungal, and anti-HIV agent. Azo compounds based on 8-hydroxyquinoline are particularly effective at chelating metal ions like Hg2+, Fe3+, Al3+, Zn2+, and Ni2+.
Despite 1-naphthylamine's lack of revealed carcinogenic potential in experiments, its production process can generate potentially harmful aromatic amines. Though established as a diazo component and intermediate in dye production, 1-naphthylamine has been linked to heavy shades on wool and cotton. 8-hydroxyquinoline and its azo derivatives have various applications, including bacteriostatic action and use as chromophoric and metallochromic indicators in analytical chemistry. 1-naphthylamine, which turns purplish-red upon exposure to air, is used in dye and rubber manufacturing and weed control.
Inspired by the "privileged structure" of 8-hydroxyquinoline, this research synthesized 8-hydroxyquinoline-based azo dyes. The experimental results demonstrate promising color fastness to wash, perspiration (both acid and alkali), and rubbing, comparable to commercially available high-performance dyes like 1-naphthylamine.
Materials & Instruments
The chemicals, α-Naphthylamine, ethanol, HCl, NaOH, 8-hydroxyquinoline, Di water, NaNO2, these are used in synthesis of dyes. DMF (Dimethylformamide). All chemicals used for the synthesis, including dispersing agent, DMSO, carrier, wetting agent, detergent, soda ash, disodium hydrogen orthophosphate dodecahydrate, and 1-histidine mono-hydrochloride monohydrate, were purchased from an Indonesian supplier named Mark Indonesian and used as received without further purification. Commercially available gray polyester fabrics were obtained from a local market for dyeing purposes.
During Synthesis of the dye, Ice bath arrangement and incubator is used. For dyeing purpose, we used IR dyeing machine and for testing purpose several machines are used. Such as, rubbing fastness machine, wash fastness testing machine, Bursting strength testing machine, Incubator, Data color Spectrophotometer, Electric Balance.
Methods
In this work, we synthesized 8-hydroxyquinoline based azo dye named hydroxy azo naphthylamine. We also dyed 100% polyester fabric with this synthesized dye. Along with dye synthesis and dyeing we also have done several tests of the dyed polyester fabric. Methods of this processes are described below:
Synthesis of Hydroxy Azo Naphthylamine: The synthesis began by dissolving 1-napthylamine and 4-chloroaniline in a mixture of ethanol and hydrochloric acid, cooled to 0°C. This temperature is crucial to prevent the desired diazonium salt from converting into an unwanted phenol byproduct. Next, a sodium nitrite solution was added slowly, and the reaction mixture was stirred for one hour at 0°C. Finally, a cold solution of 8-hydroxyquinoline and sodium hydroxide was added gradually. After another hour of stirring at 0°C, the crude product was filtered, washed, and dried. Recrystallization from ethanol yielded deep red crystals of the desired azo dye with a good yield of 83% (Figure 1) (Figure 2).
Dyeing of polyester fabric: The synthesized dyes were used to dye 100% polyester fabric using two methods: with and without a carrier. Both methods used a 4% dye concentration and a 1:40 liquor ratio in an IR dyeing machine. Two separate dye baths were prepared. One bath contained 2% dispersing agents, 2% carrier, 1 g/L wetting agent, and 1 g/L leveling agent, while the other bath contained only 2% dispersing agents, 1 g/L wetting agent, and 1 g/L leveling agent. Each bath was then used to dye one of the fabric samples. The dyeing process took place in a closed bath at 95°C for 60 minutes. After dyeing, the samples were washed with hot water (50°C) for 10 minutes, followed by cold water at room temperature for another 10 minutes. Finally, the samples were dried and cured at 150°C for 10 minutes and then at 180°C for 2 minutes (Figure 3).
The dyeing cycle is shown in Figure 4.
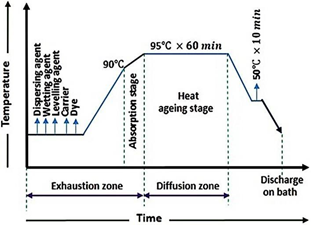
Figure 4 A schematic diagram showing the dyeing cycle of PET fabric with the as-synthesized dispersed dyes.
Color fastness test
Fastness to wash: A small fabric sample (10 cm x 4 cm) was sewn along all four edges with the same size piece of multi-fiber fabric. This sewn specimen was then washed in a special washing machine called a gyro-wash machine. The washing conditions were: a temperature of 60°C, a washing time of 30 minutes, a detergent solution concentration of 4 g/L, a soda ash concentration of 1 g/L, and a fabric-to-liquor ratio of 1:50. After washing, the specimen was evaluated for two things: any change in its color and any staining of the adjacent multi-fiber fabric. This evaluation was done using a standard grayscale method specified in the ISO 105 C06 A2S test method.
Fastness to rubbing: To test the color fastness against rubbing, square fabric samples measuring 5 cm x 5 cm were cut. (Image of a 5 cm x 5 cm fabric sample). Each sample was mounted onto the platform of a crockmeter, a device specifically designed for this test. (Image of a crockmeter). Additionally, a 5 cm x 5 cm white bleached fabric sample was mounted onto the finger of the crockmeter. (Image of a white bleached fabric sample). This white sample was then rubbed against the colored fabric sample for 10 seconds, repeated for a total of 10 cycles. After rubbing, the effect on the colored fabric sample was assessed using a grayscale according to the ISO 105 X12 2002 standard. (Image of a grayscale chart). This assessment helps determine how much color has been transferred from the colored fabric to the white fabric due to rubbing.
Fastness to perspiration
Fastness to acid perspiration: A dyeing solution was prepared containing sodium chloride, disodium hydrogen orthophosphate dodecahydrate, L-histidine mono hydrochloride monohydrate, and acetic acid. The solution was adjusted to an acidic pH of 5.5. A fabric sample (10cm x 4cm) was immersed in the solution, covered with a white fabric, and tied securely. The sample was then heated in an oven at 32°C for 4 hours. After cooling, the sample was untied and evaluated for staining using a standard grayscale method.
Fastness to alkali perspiration: This test was performed similarly to the acid perspiration test, but with an alkaline environment. To achieve this, 0.1 N sodium hydroxide (NaOH) was dissolved in 100 ml of distilled water. The same methods and procedures used in the alkali perspiration test were followed, and the results were recorded using a grayscale according to ISO 105 E04 1994.
Bursting strength test: When pressure is applied to fabric, it stretches in all directions simultaneously. As the pressure increases, the fabric eventually reaches a point where it can no longer withstand it and bursts. This point is known as the bursting strength.
Bursting strength of fabric: Measured in pounds per square inch (psi) or kilograms per square centimeter (kg/cm^2), bursting strength is a vital property for parachute fabrics. It directly impacts the parachute's ability to safely slow down a falling object, ensuring a safe landing.
Several factors influence the bursting strength of a fabric:
The bursting strength of a parachute fabric is crucial for ensuring the safety of its users. Manufacturers can design and produce safe and reliable parachutes for various applications by understanding the factors that affect this property.
Bursting strength tester: A bursting strength tester measures the force needed to burst a material stretched across a rubber diaphragm. During testing, the fabric fails in the direction with the lowest breaking extension, not necessarily the direction with the absolute weakest strength. This is because the burst test applies equal pressure in all directions, forcing the direction with the least stretch to break first. Imagine a fabric sheet stretched taut like a drumhead. If you poke it with a finger, it will tear along the direction with the least resistance, even if another direction has weaker fibers overall. This is analogous to how a fabric fails during a burst test.
Testing procedure: Hydraulic bursting strength testers measure the force required to burst a fabric sample using hydraulic pressure. This method involves inflating a rubber diaphragm with increasing pressure, causing the fabric specimen to stretch and eventually rupture.
Test procedures:
Color Matching Cabinet (CMC)
CMC, short for Color Matching Cabinet, is a test used to measure the difference in color between a standard swatch and bulk-dyed fabric. It's also known as the DE Test or DATA COLOR RESULT. Sometimes, it's difficult to accurately judge the differences in a fabric's tone, depth, and even its appearance under different lighting conditions (metamerism) with just our eyes. This can lead to inconsistencies in color between the desired standard and the actual production fabric.
Enter the CMC Test: This test utilizes a spectrophotometer to objectively measure the color of both the standard swatch and the production fabric. The instrument then generates a DE value, representing the quantitative color difference between the two samples.
Here's how it works:
Spectrophotometer: A spectrophotometer is a physical tool that is used by dyeing factories and colorant Manufacturers all over the world. Normally, the color lab manager analyzes the swatch's color with a spectrophotometer's help (Figure 6).
Explanation of some symbolic meaning
L*: The color quality of lightness or darkness.
A*: Red/ green coordinate.
B*: Yellow/blue coordinate.
C*: Chroma is the quality of a color’s purity, intensity or saturation. Vivid or dulls identified as saturation.
H*: The common distinction between colors position around a color wheel is called hue (Figure 7).
|
DE/Delta E (Color difference) |
DL*/Delta L |
|
|
➢ Pass/fail/warning depends on this value. |
Difference in lightness / darkness value. |
|
|
➢ Shade not ok if DE result not pass. |
+DL* = lighter shade. |
|
|
➢ It is also called Total color difference. |
-DL* = darker shade. |
|
|
➢ DE = 0 TO 0.75 = PASS |
||
|
= 0.75 TO 1 = WARN (Commercially Pass) |
||
|
= Above 1 = FAIL |
||
|
Da*/Delta a |
Db*/ Delta b |
DC* |
|
Difference on red/green axis. |
Difference on yellow/blue axis. |
Difference in chroma. |
|
+Da* = redder. |
+Db* = yellower |
+DC* = brighter. |
|
-Da* = greener |
-Db* = bluer |
-DC* = duller. |
Results of synthesis of hydroxy azo naphthylamine
We got a dye from the synthesis of 1-napthylamine. By distinguishing the physical appearance of dyes like odor, colors are same as our expectation. The dye particle size is in proper granular form as the dye penetrates in fabric surface properly (Figure 8).
Moreover, we maintain the proper temperature so that diazonium salt formed properly because it converted into phenol if the temperature is greater than 10 c and finally diazonium salt reacts with 8hydroxyquinoline and formed our desired dye Hydroxy Azo Naphthylamine.
This synthesized dyes, were in granular form. Then By using mortar, the finer form of dye are obtained and filter it in a cup. Otherwise, dyes can’t penetrate properly in the fabric (Figure 9).
Polyester fabric’s dyeing result
The dyeing result in continuous method using different chemicals is shown in figure. The appearance of the dyed fabric as shown in the figure which is uneven dyed fabric (Figure 10).
When we mix the dye in the solution, we notice some abnormal coagulation od dye particles in the solution. To get rid of this problem, we have been stirring the solution for a long time. But the problem remained that’s why uneven dyeing is formed in the fabric (Figure 11).
Color fastness to wash result
From the experiment we got the result of wash fastness of the dyed fabric. It is demonstrated that the color change rating of the dyed fabric is similar with each other of the dyed fabrics but color staining properties in case of polyester, acetate, and nylon showed better result when fabrics are colored in exhaust method. The result of color change and color staining is quite satisfactory, as the results are very closer as per the requirements of the international brands. The average rating of wash fastness of the fabric in 4 to 5 that means the wash fastness is very good (Figures 12–14) (Table 1).
Fabric |
Rating |
Acetate |
4-5 |
Cotton |
4 |
Nylon |
4 |
Polyester |
5 |
Acrylic |
5 |
Wool |
4-5 |
Table 1 Wash fastness rating for different fabric
Color fastness to rubbing result
The result of rubbing fastness properties of dyed fabric are shown above. From the above evaluation scale. We can put the rating of rubbing fastness. Comparing with the evaluation scale we can say that the dry rubbings rating is 4 to 5, which means that the rubbing fastness of the dyed fabric in dry state is very good. And for the wet rubbing we can evaluate that the fastness rating is 3 to 4, which refers that the rubbing fastness of the dyes fabric is moderate to good (Figures 15–17) (Table 2).
Fabric condition (rubbing) |
Rating |
Dry |
4-5 |
Wet |
3-4 |
Table 2 Rubbing fastness result
Color fastness to perspiration result
In this experiment we have done the test of color fastness to perspiration for both acidic and alkaline perspiration. For both acidic and alkaline perspiration. We got similar result. For acetate and cotton fabric, the fastness rating is 4, which refers good fastness. For nylon, acrylic and wool, the fastness rating is 4 to 5 which refers very good fastness. And for polyester fabric, the rating is 5 which refers the excellent fastness of the dyed fabric (Figure 18) (Table 3).
Fabric |
Rating |
Acetate |
4-5 |
Cotton |
4 |
Nylon |
4 |
Polyester |
5 |
Acrylic |
5 |
Wool |
4-5 |
Table 3 APerspiration (acidic & alkaline) fastness rating for different fabric
Bursting strength tester result
This figure represents values of bursting strength of grey and dyed fabric. For grey and dyed stage respectively, bursting strength 352 kPa at 137 GSM and 335 kPa at 144 GSM.
In case the dyed fabric GSM is higher than grey fabric due to application of dyes, chemicals and auxiliaries. But the actual structure of fabric is hampered and loses its strength. In the stage after dyeing, strength of dyed fabric decreases (Figure 19).
CMC result
Here, in this section we distinguish the color matching cabinet in two ways. The one of the ways is taking the grey fabric (Figure 21a) as swatch to get CMC result of darker shade dyed fabric sample.
Again taking grey fabric as swatch (Figure 21b) to get CMC result of lighter shade dyed fabric sample. The other ways are one lighter shade dyed fabric sample (Figure 21c) taking as swatch to get CMC result for another darker shade of dyed fabric sample (Figure 20) (Figure 21).1–8
New azo dyes based on 8-hydroxyquinoline were successfully synthesized and tested for dyeing polyester (PET) fabrics. These dyes showed good affinity for PET, resulting in moderate to excellent washing and light fastness, and very good rubbing and perspiration fastness. The dyes performed comparably to commercially available high-performance disperse dyes, including those based on anthraquinone. Spectrophotometer analysis confirmed these findings, indicating good color properties. This research suggests that 8-hydroxyquinoline-based azo dyes offer a promising and cost-effective alternative for dyeing PET fabrics. They challenge conventional methods and add value to the PET industry by simplifying the dyeing process. The introduction of hydroxy azo naphthylamine dyes marks a potential new era in PET dyeing, opening doors for further research and development of environmentally friendly alternatives. While conventional disperse dyeing remains the dominant technique, 8-hydroxyquinoline-based azo dyes present a promising new avenue for the future of PET fabric dyeing. This research paves the way for further exploration and optimization of this novel method, potentially leading to more sustainable and environmentally responsible dyeing practices.
None.
None.
Authors declare that there is no conflict of interest.

©2023 Anik, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.