Journal of
eISSN: 2574-8114


Research Article Volume 2 Issue 6
1College of Textiles, Donghua University, Shanghai, China
2Key Laboratory of Textile Science & Technology, Ministry of Education, China
3Insititute of Bast Fiber Crops, Chinese academy of agricultural sciences, China
Correspondence: Chongwen Yu, College of Textiles, Donghua University, Key Laboratory of Textile Science & Technology, Ministry of Education, Shanghai, 201620, Institute of Bast Fiber Crops, Chinese academy of agricultural sciences, Changsha, Hunan, China, Tel 13651603285
Received: July 27, 2017 | Published: August 31, 2017
Citation: Meng C, Yu C. The use of reductants in oxidation degumming of ramie. J Textile Eng Fashion Technol. 2017;2(6):511-516. DOI: 10.15406/jteft.2017.02.00077
When ramie fiber was extracted by oxidation degumming, large amount of hydroxyl groups were converted to carboxyl groups and aldehydes groups. This would do great damage to tensile properties of fiber, for the hydrogen bonds among carboxyl groups and aldehydes groups were much weaker than the ones among hydroxyl groups. In this study, tensile properties of fiber extracted by oxidation degumming were improved by reducing the fiber in solutions containing reductants. Three kinds of reductants, including thiourea dioxide, vitamin C, sodium hydrosulfite, were investigated in this study and L9(34) orthogonal design was used to find the optimal reaction condition for each reductant. Results showed that thiourea dioxide and sodium hydrosulfite should be used under the pH value of 11.0~12.0; and vitamin C should be used under the pH value of 6.0~7.0. The optimal reaction condition for thiourea dioxide, vitamin C, sodium hydrosulfite, were 6%, 100˚C, 20min; 6%, 20˚C, 20min; 6%, 100˚C, 20min, respectively. After reducing in thiourea dioxide, vitamin C, sodium hydrosulfite solutions, and the tensile strength of fiber increased by 29.08%, 27.86%, 3.2%, respectively.
Keywords: ramie fiber, oxidation degumming, reductants, tensile property
Ramie is a perennial herb whose fiber could be used as excellent materials for clothing fabrics, fiber reinforced composites, car accessories, ect.1,2 In China, ramie is one of the main economic crops; the production of ramie in China has accounted more than 90% of the total yield in the world.3 The production made of ramie fiber possesses many excellent properties, such as high moisture absorption capacity, good thermal conductivity, outstanding antibacterial function and favorable air permeability.4,5 Cellulose is the main component of ramie fiber, while the other components in ramie, such as pectin, lignin, water soluble, etc, are defined as gums.6 Degumming refers to the removal of heavily coated gummy material from the cellulosic part of plant fiber, and it is necessary prior to further spinning process.7 There were mainly two approaches of ramie degumming, namely, traditional chemical degumming and bio-degumming.8 The energy and time consumption, chemical oxygen demand (COD value) of degumming wastewater in traditional chemical degumming was rather high, for cellulose fiber was extracted by scouring raw ramie in concentrated NaOH under high pressure for 6h to 8h.9 Bio-degumming is an eco-friendly way of ramie fiber extraction; however, the harsh reaction condition and sophisticated equipment inhibited its further industrial application.10 Under this circumstance, novel chemical degumming method ‘oxidation degumming’ showed good application foreground, for it can get high degumming yield under low energy consumption in only 4h.8
Compared with traditional degumming, oxidation degumming with H2O2 is effective, eco-friendly, and of high fiber yield.9 H2O2 can decompose into several kinds of free radicals (such as O-2·, OH·, OOH·, OH-, ect) in alkali condition, these radicals have strong oxidizing ability. The gummy materials have a relatively lower degree of polymerization and crystallinity which is easily attacked by these radicals and are easily dissolved in degumming solution. However, cellulose can resist alkaline condition, thus it can be separated from raw ramie.10 Due to the strong oxidation ability of the peroxides degumming solution, great amount of cellulose degradation may occur during the degumming process and large proportion of hydroxyl groups in cellulose were converted to acid groups (carboxyl groups and aldehyde groups), which would cause great damage to fiber properties.11 In order to address this issue, oxidation degumming has been extensively studied for many years. Liu10 improved tensile properties of oxidation degummed ramie fiber by adding H2O2 stabilizer in degumming solution. Meng12 protected cellulose successfully by adding anthraquinone in degumming solution. Li13 controlled the decomposition speed of H2O2 by multiple feeding NaOH and H2O2 in degumming solution. However, most of these methods focused on preventing cellulose from degradation, the study on acid groups were seldom studied. Li14 tried to convert the acid groups in oxidation degummed ramie fiber back to hydroxyl groups by reducing the fiber in NaH4B solution, and results showed that this process was very helpful in improving the tensile properties of fiber, However, this important process has not been studied sufficiently yet.
In this paper, several kinds of reductants, including vitamin C, sodium hydrosulfite, thiourea dioxide, and sodium hydrogen sulfite was used in oxidation degumming of ramie. The optimal reaction condition of these reductants was studied respectively and the properties of fiber reduced by various reductants were compared.
Materials
In this experiment, the raw ramie was obtained from Changde, Hunan Province, China. The chemical composition of the ramie material was tested and listed in Table 1.
Ingredient |
Cellulose |
Hemicellulose |
Pectin |
Lignin |
Wax |
Ash |
Water Solubles |
Content (%) |
74.25 |
13.8 |
5.16 |
1.3 |
1.04 |
1.05 |
3.4 |
Table 1 Chemical composition of raw ramie
Chemicals
The main chemicals used in this study were H2O2, NaOH, Na5P3O10, HEDP, vitamin C (C6H8O6), sodium hydrosulfite (Na2O4S2), thiourea dioxide (CH4N2O2S), which were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). All chemicals used in this study were analytical grade.
Process for the degumming of ramie
Degumming solution was composed of 6% (o.w.f) H2O2, 10% (o.w.f) NaOH, 4% (o.w.f) Na5P3O10, 2% HEDP, with a liquor ration of 1:10. Raw ramie was immersed in the degumming solution, and scoured under the temperature of 85˚C for 60 minutes. Then the temperature was raised to 125˚C (with pressure of 0.6kg), and kept for another 60 minutes.
Subsequently, the treated fibers were immersed in solution composed of various reductants for the reducing process. L9(34) orthogonal design was used to investigate the optimal reaction condition for thiourea dioxide (Table 2), vitamin C (Table 3), sodium hydrosulfite (Table 4).
Levels |
Dosage (%) |
Temperature (˚C) |
Time (min) |
1 |
2 |
60 |
20 |
2 |
4 |
80 |
40 |
3 |
6 |
100 |
60 |
Table 2 Variables and levels of orthogonal design of CH4N2O2S
Levels |
Dosage (%) |
Temperature (˚C) |
Time (min) |
1 |
2 |
20 |
20 |
2 |
4 |
40 |
40 |
3 |
6 |
60 |
60 |
Table 3 Experimental range and levels of independent variables of Vitamin C
Levels |
Dosage (%) |
Temperature (˚C) |
Time (min) |
1 |
2 |
60 |
20 |
2 |
4 |
80 |
40 |
3 |
6 |
100 |
60 |
Table 4 Variables and levels of orthogonal design of Na2O4S2
Finally, the fiber was washed thoroughly with distilled water and properly dried at oven (100˚C, 3h) for the subsequent characterization.
Mechanical property test
Fibers samples were conditioned in standard atmospheric condition (T=20˚C±2˚C, RH=65%±2%) for 24h before the mechanical test. Breaking strength, breaking elongation was tested using a XQ-2 fiber strength instrument under the condition of 20˚C and RH 65%. The pre-tension was 0.3 cN/dtex. The clamping distance was set with 20mm, and the descending speed of the bottom clamp was 20mm/min.
ORP value
Oxidation reduction potential (ORP)13 is an important water chemistry parameter and it provides a measurement tool for oxidizing or reducing capacity of the ambient water. ORP is measured in volts (V) or millivolts (mV) with oxidation–reduction potentiometer. The more positive the potential value, the greater the species’ affinity for electrons and tendency to oxidize.
The relationship between ORP and the concentrations of the oxidized and reduced forms of a substance is given by Nernst Equation (1),
(1)
Where Eh is the potential at the standard hydrogen electrode (mV), E0 is the standard potential of the system when the activities of all reactants are unity, R is the universal gas constant (8.314 JK−1/ mol), T is the absolute temperature in Kelvin, F is the Faraday constant (96.5 JK−1/ mol), n is the number of electrons involved in reaction, [Ox] is the chemical activity for the oxidant, [Red] is the chemical activity for the reluctant.
In this experiment, the ORP value of the degumming solution were also determined and monitored by MODEL 421 ORP meter (Dapu Instrument, Shanghai, China).
The reducing ability of reductants
ORP values reflected the comprehensive oxidation ability of degumming solution. Solutions with positive ORP values exhibited oxidability and solution with stronger oxidability got higher ORP values; solutions with negative ORP values exhibited reducibility and solution with stronger reducibility got higher absolute ORP values.
It was wildly known that the reducibility of reductants vary with their pH value of solution, in order to searching for the optimal pH values for reducing reaction, the ORP values of reducing solution composed of 2% (o.w.f.) CH4N2O2S, 2% (o.w.f.) Na2O4S2 and 2% (o.w.f.) C6H8O6 was tested respectively (Table 5). It was obvious from Table 5, the ORP value of CH4N2O2S solution was -120mV under the pH value of 7.0~8.0, however, the ORP value decreased to -720mV under the pH value of 11.0~12.0. It could be deduced that pH value have strong influence on the reducibility of CH4N2O2S and this reductant should be used under alkali condition. The ORP value of Na2O4S2 solution was -540mV under the pH value of 7.0~8.0 and decreased to -650mV under the pH value of 11.0~12.0, which proved that pH value showed some influence on the reducibility of Na2O4S2 and it should be used under alkali condition. C6H8O6 can only be used under pH value of 6.0~7.0, for C6H8O6 was easily to be damaged under alkali condition. The ORP value of C6H8O6 solution was -100mV.
pH Value |
ORP Value |
|
CH4N2O2S |
7.0~8.0 |
-120 |
11.0~12.0 |
-720 |
|
Na2O4S2 |
7.0~8.0 |
-540 |
11.0~12.0 |
-650 |
|
NaHSO3 |
7.0~8.0 |
-140 |
11.0~12.0 |
-140 |
|
C6H8O6 |
6.0~7.0 |
-100 |
Table 5 The reducing ability of reductants
The effect of various reductants on tensile property of fiber
Sodium hydrosulfite: L9(34) orthogonal design was used to investigate the optimal reaction condition of CH4N2O2S, and the results of tensile properties were shown in Table 6. Tensile strength of fiber increased with CH4N2O2S dosage, reaction temperature and reaction time (Figure 1A), for the reducibility of CH4N2O2S solution was strongly boosted under such condition. Tensile elongation of fiber increased with CH4N2O2S dosage, reaction temperature and reaction time, from 2%~4%, 60˚C ~80˚C, 20min~40min; further increase of these parameters would cause decrease of fiber elongation (Figure 1B), which indicated relatively mild reaction condition was good for improving elongation of fiber. ANOVA analysis (Table 7) revealed that CH4N2O2S dosage, reaction temperature and reaction time had significant effect on tensile strength of fiber; however, these three parameters did not have significant effect on tensile elongation of fiber. Therefore, when tensile properties and degumming efficient was both taken into consideration, the optimal reaction condition of CH4N2O2S was 6%, 100˚C, 20min.
S.No |
Dosage (%) |
Temperature (˚C) |
Time (min) |
Tensile Strength (cN) |
Tensile Elongation (%) |
1 |
1 |
1 |
1 |
38.37 |
2.66 |
2 |
1 |
2 |
2 |
54.82 |
3.24 |
3 |
1 |
3 |
3 |
66.26 |
2.61 |
4 |
2 |
1 |
3 |
46.37 |
2.65 |
5 |
2 |
2 |
1 |
60.15 |
2.66 |
6 |
2 |
3 |
2 |
68 |
2.37 |
7 |
3 |
1 |
2 |
50.14 |
2.81 |
8 |
3 |
2 |
3 |
62.84 |
2.37 |
9 |
3 |
3 |
1 |
68.39 |
2.86 |
Table 6 Tensile strength and elongation of fiber in orthogonal design of CH4N2O2S
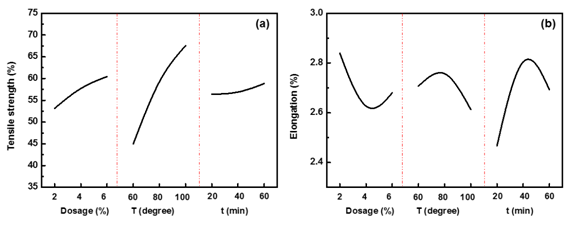
Figure 1 The effect of CH4N2O2S dosage, reaction temperature and reaction time on tensile properties of fiber: (a) Strength; (b) Elongation of fiber.
Tensile Strength (cN) |
Elongation (%) |
|||||||||
SS |
Df |
F |
F0.05(2,2) |
S |
SS |
Df |
F |
F0.05(2,2) |
S |
|
D (%) |
146.39 |
2 |
377.29 |
19 |
* |
0.12 |
2 |
1.06 |
19 |
|
T(˚C) |
964.73 |
2 |
2486.42 |
19 |
* |
0.03 |
2 |
0.29 |
19 |
|
t(min) |
22.84 |
2 |
58.86 |
19 |
* |
0.3 |
2 |
2.79 |
19 |
|
Error |
0.39 |
2 |
0.11 |
2 |
||||||
Table 7 The ANOVA analysis of orthogonal design of CH4N2O2S
Vitamin C: L9(34) orthogonal design was used to investigate the optimal reaction condition of vitamin C, and the results of tensile properties were shown in Table 8. Tensile strength of fiber increased with Vitamin C dosage and decreased with reaction temperature, however, reaction time did not show much influence on tensile strength. That was because the efficiency and speed of reducing reaction of vitamin C was high; however, vitamin C was easily destroyed under higher temperature (Figure 2A). Tensile elongation of fiber increased with vitamin C dosage, reaction temperature and reaction time, from 2%~4%, 20˚C ~ 60˚C, 20min~40min; further increase of these parameters would cause decrease of fiber elongation (Figure 2B), which indicated relatively mild reaction condition was good for improving elongation of fiber. ANOVA analysis (Table 9) revealed that vitamin C dosage, reaction temperature had significant effect on tensile strength of fiber, and however, all of the three parameters did not have significant effect on tensile elongation of fiber. Therefore, when fiber property and degumming efficient was both taken into consideration, the optimal reaction condition of vitamin C was 6%, 20˚C, 20min.
S.No |
Dosage (%) |
Temperature (˚C) |
Time (min) |
Tensile |
Tensile |
1 |
1 |
1 |
1 |
40.14 |
2.076 |
2 |
1 |
2 |
2 |
34.28 |
2.262 |
3 |
1 |
3 |
3 |
32.13 |
2.284 |
4 |
2 |
1 |
3 |
46.43 |
2.117 |
5 |
2 |
2 |
1 |
40.25 |
2.201 |
6 |
2 |
3 |
2 |
38.26 |
2.14 |
7 |
3 |
1 |
2 |
48.95 |
2.146 |
8 |
3 |
2 |
3 |
43.55 |
2.357 |
9 |
3 |
3 |
1 |
40.18 |
2.451 |
Table 8 Tensile strength and elongation of fiber in orthogonal design of Vitamin C

Figure 2 The effect of Vitamin C dosage, reaction temperature and reaction time on tensile properties of fiber: (a) Strength; (b) Elongation of fiber.
Tensile Strength (cN) |
Elongation (%) |
|||||||||
SS |
Df |
F |
F0.05(2,2) |
S |
SS |
Df |
F |
F0.05(2,2) |
S |
|
D (%) |
120.1 |
2 |
300.24 |
19 |
* |
0.043 |
2 |
4.78 |
19 |
|
T(˚C) |
109.23 |
2 |
273.07 |
19 |
* |
0.058 |
2 |
6.44 |
19 |
|
T(min) |
0.19 |
2 |
0.47 |
19 |
0.012 |
2 |
1.33 |
19 |
||
Error |
0.4 |
2 |
0.01 |
2 |
||||||
Table 9 The ANOVA analysis of orthogonal design of Vitamin C
Sodium hydrosulfite: L9(34) orthogonal design was used to investigate the optimal reaction condition of Na2O4S2, and the results of tensile properties were shown in Table 10. Tensile strength of fiber increased with Na2O4S2 dosage and reaction temperature, however, reaction time did not show much influence on tensile strength, for the reducibility of Na2O4S2 solution was strongly boosted under such condition (Figure 3A). Tensile elongation of fiber increased with Na2O4S2 dosage, reaction temperature and reaction time, from 2%~4%, 60˚C~80˚C, 20min~40min; further increase of these parameters would cause decrease of fiber elongation (Figure 3B), which indicated relatively mild reaction condition was good for improving elongation of fiber. ANOVA analysis (Table 11) revealed that Na2O4S2 dosage, reaction temperature had significant effect on tensile strength of fiber, and however, all of the three parameters did not have significant effect on tensile elongation of fiber. Therefore, when fiber property and degumming efficient was both taken into consideration, the optimal reaction condition of Na2O4S2 was 6%, 100˚C, 20min.
S.No |
Dosage (%) |
Temperature (˚C) |
Time (min) |
Tensile |
Tensile |
1 |
1 |
1 |
1 |
38.38 |
2.75 |
2 |
1 |
2 |
2 |
48.75 |
2.66 |
3 |
1 |
3 |
3 |
60.14 |
2.47 |
4 |
2 |
1 |
3 |
43.37 |
2.76 |
5 |
2 |
2 |
1 |
57.23 |
3.03 |
6 |
2 |
3 |
2 |
65.89 |
2.7 |
7 |
3 |
1 |
2 |
49.44 |
2.6 |
8 |
3 |
2 |
3 |
61.4 |
2.47 |
9 |
3 |
3 |
1 |
67.23 |
2.87 |
Table 10 Tensile strength and elongation of fiber in orthogonal design of Na2O4S2
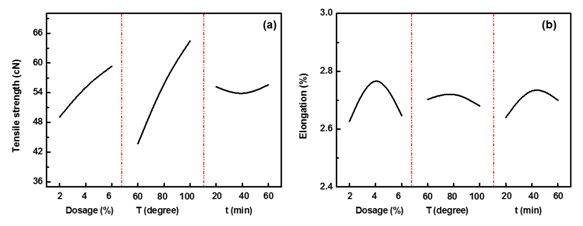
Figure 3 The effect of Vitamin C dosage, reaction temperature and reaction time on tensile properties of fiber: (a) Strength; (b) Elongation of fiber.
Tensile Strength (cN) |
Elongation (%) |
|||||||||
SS |
Df |
F |
F0.05(2,2) |
S |
SS |
Df |
F |
F0.05(2,2) |
S |
|
D (%) |
161.35 |
2 |
223.17 |
19 |
* |
0.0075 |
2 |
0.466 |
19 |
0 |
T(˚C) |
648.02 |
2 |
896.29 |
19 |
* |
0.002 |
2 |
0.012 |
19 |
0 |
t(min) |
10.77 |
2 |
14.89 |
19 |
0.023 |
2 |
0.143 |
19 |
0 |
|
Error |
0.72 |
2 |
0.16 |
2 |
0 |
|||||
Table 11 The ANOVA analysis of orthogonal design of Na2O4S2
Comparison of the three reductants
In order to compare the effect of these reductants, ramie fiber was reduced in thiourea dioxide (6%, 100˚C, 20min), vitamin C (6%, 20˚C, 20min), sodium hydrosulfite on fiber property (6%, 100˚C, 20min), and the results were shown in Table 12. It was obvious that the fiber reduced with thiourea dioxide and sodium hydrosulfite got similar tensile properties, which was % and % higher than that reduced with vitamin C. That was because the reductivity of vitamin C was not as strong as thiourea dioxide and sodium hydrosulfite, moreover, vitamin C tended to loss its activity because of the metal ions existed in the degumming solution. After reducing in thiourea dioxide, vitamin C, sodium hydrosulfite solutions, and the tensile strength of fiber increased by 29.08%, 27.86%, 3.2%, respectively.
Tensile Strength (cN) |
Tensile Elongation (%) |
|
Thiourea Dioxide |
68.39 |
2.86 |
Sodium Hydrosulfite |
67.23 |
2.87 |
Vitamin C |
50.11 |
2.2 |
Before reducing |
48.5 |
2.1 |
Table 12 Tensile properties of fiber reduced with thiourea dioxide, vitamin C, and sodium hydrosulfite.
In this study, tensile properties of fiber extracted by oxidation degumming were improved by reducing the fiber in solutions containing reductants. Three kinds of reductants, including thiourea dioxide, vitamin C, sodium hydrosulfite, were investigated in this study and L9(34) orthogonal design was used to find the optimal reaction condition for each reductant. Results showed that thiourea dioxide and sodium hydrosulfite should be used under the pH value of 11.0~12.0; and vitamin C should be used under the pH value of 6.0~7.0. The optimal reaction for thiourea dioxide, vitamin C, sodium hydrosulfite, was 6%, 100˚C, 20min; 6%, 20˚C, 20min; 6%, 100˚C, 20min, respectively. After reducing in thiourea dioxide, vitamin C, sodium hydrosulfite solutions, and the tensile strength of fiber increased by 29.08%, 27.86%, 3.2%, respectively. That was because the carboxyl groups and aldehyde groups in cellulose were reduced to hydroxyl groups, which can generate stronger hydrogen bonds and thus improve the tensile properties of fiber.
The authors acknowledge the financial support from the earmarked fund for China Agriculture Research System for Bast and Leaf Fiber Crops: CARS-19.
China Academy of Agricultural Science and Technology Innovation Project: ASTIP-IBFC07.
The innovation fund for graduate students in Donghua University: 16D310107.
Author declares there is no conflict of interest.

©2017 Meng, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.