Journal of
eISSN: 2379-6359


Research Article Volume 6 Issue 6
1Department of Otorhinolaryngology Ain Shams University Cairo, Egypt
2Department of Pathology Ain Shams University Cairo, Egypt
Correspondence: Badr E Mostafa 75 El Nozha Street Heliopolis 11361 Cairo, Egypt, Tel +2012 27461137
Received: August 10, 2016 | Published: April 17, 2017
Citation: Mostafa BE, Fawaz S, Taha MS, Salman M (2017) Ultrastructural Analysis of the Junctional Zone of Vocal Fold Polyps and Nodules. J Otolaryngol ENT Re 6(6): 00182. DOI: 10.15406/joentr.2017.06.00182
Objectives: To study the ultrastructural changes of the junctional zone the apparently normal mucosa of vocal fold polyps and nodules.
Methods: 15 lesions (nodules and polyps) by standard microscopy (Hx. and eosin and PAS) and by electron microscopy
Results: The junctional zone showed basement membrane thickening and the vessels showed endothelial interruptions. Some endothelial cells were degenerated and a few were apoptotic. Endothelial cells showed increased microfilaments with foci of detachment from the underlying basement membrane. Larger blood vessels showed large plump endothelial cells alternating with attenuated ones. There was also widening of the intercellular junctions between the endothelial cells.
Conclusion: Our findings indicate that the pathological changes in the substance of vocal fold polyps and nodules extend beyond the gross visible edges of the lesion. This may have implications in the planning of surgical excision to remove all potentially unstable tissue to prevent recurrences.
Keywords: larynx, dysphonia, vocal nodules, vocal polyps, phonosurgery, ultrastructural, junctional, anteroposterior, food, surgical
Vocal nodules and polyps are common causes of voice disorders. The differentiation between vocal nodules and polyps is not clear cut, and the issue is further confused by differences in the terminology used by the laryngologist and pathologist.1 Polyps and nodules of vocal cords are considered to be tissue reactions to chronic mechanical irritations in the form of vocal misuse or abuse,2 The main difference is usually unclear3 but may be in the time course with polyps being considered younger lesions and nodules of more long standing nature.4 The etiology and pathogenesis of the vocal disorders have not been elucidated. Various histological and biochemical mechanisms have been reported including the involvement of nitric oxide, hormones, or vascular endothelial disturbances.1,5‒7 Therapy for such lesions is usually a combination of voice therapy and surgical excision. The basic principle of phonosurgery is to maintain or improve the functional structure of the vocal fold by respecting its layered structure. This is achieved by means of minimal tissue excision, minimal disruption of the superficial layer of the lamina propria and preservation of the epithelium, especially at the vibrating edge.8
The aim of this study is to determine the changes of the transitional zone (the angle between apparently normal mucosa and the base of the polyp or nodule) to determine how far do the pathological changes extend along the free edge of the vocal fold.
The study was conducted in a tertiary referral University Hospital. It included 15 lesions (seven nodules and 8 polyps). The lesions were microsurgically excised from 15 patients (7 females and 8 males) with a median age of 36years. The specimens included the nodule or the polyp and the adjacent mucosa (transitional zone). The transitional zone mucosa was specifically studied. The specimens were bisected. One half was preserved in formalin, then processed and embedded in paraffin for haematoxylin and eosin and PAS staining. The second half was preserved in F/G solution then processed and embedded in epoxy resin. Ultrathin sections were cut and stained by uranyl acetate and lead citrate and then examined by Transmission Electron Microscope Philips 400 (at 80K.V.)
Histological findings
The basement membrane was thickened (PAS +ve), with increased vascularity. Two types of vessels were found. Vessels with very thin interrupted endothelial lining and no basal lamina around; and much thicker vessels with interrupted multi-layered basement membrane. There was also stromal oedema and foci of interstitial haemorrhage and PAS +ve stained hyaline material (Figure 1).
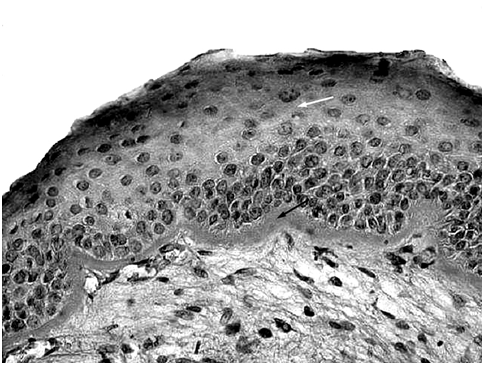
Figure 1 Light microscopic section of the junctional zone of a vocal fold polyp showing the hyperplastic epithelium (white arrow) resting on thickened PAS positive basement membrane (black arrow). (PAS x400).
Electron microscopic findings
Epithelium: Marked intercellular gapping with interrupted basal lamina with focal deposition of basement membrane like material in wide areas of the sub-epithelial layer (Figure 2 & 3).

Figure 2 Electron micrograph showing parts of two adjacent basal epithelial cells with slightly widened intercellular spaces (arrow), a normal nucleus (N) and multilayered disrupted basal lamina (BL). (EMx6000).

Figure 3 Electron micrograph showing parts of adjacent epithelial cells with marked widening of intercellular spaces (arrow) (EM x4600).
Blood vessels: Small blood vessels showed marked thinning of the endothelial lining cells. Some endothelial cells were degenerated and a few were apoptotic (Figure 4). Endothelial cells showed increased microfilaments especially in the lateral zones with foci of detachment from the underlying basement membrane. Multi layering of the pericapillary basement membrane was also noted with focal irregularity. Larger blood vessels showed large plump endothelial cells alternating with attenuated ones. There was also widening of the intercellular junctions between the endothelial cells (Figure 5). Both the plump endothelial cells and especially the attenuated ones were widely separated and detached from the underlying multilayered basement membrane. The latter showed foci of disruption and loss. Interstitial hemorrhage and collagen deposition were also noted (Figure 6).

Figure 4 Apoptotic change in endothelial cells and marked collagen deposition in interstitial tissue. [EM x7200].
Vocal fold polyps and nodules are non neoplastic lesions that arise mostly in response to vocal fold trauma (abuse or misuse of voice), but not all the voice abusers develop vocal fold polyp or nodule. The pathogenetic cause and the extent of damage to the mucosa are still debatable. Repeated microtrauma to the vocal folds may lead to basement membrane zone disruption and injury to the superficial layer of the lamina propria. The injury, if repetitive, leads to aberrant healing and a fibroblastic response involving increased fibronectin deposition.9 In addition circulation impediment, thrombosis, exudation and edema of the lamina propria of the mucous membrane due to inflammation, with secondary atrophy of the epithelium of the fold.4,5 The central pathogenic pathways seems to involve trauma to the blood vessels and an inflammatory reaction generated by vascular leak.1,10,11
In the present study, we studied the structural and ultra structural changes occurring in the transitional zone between the lesion polyp or nodule and the apparently unaffected mucosa of the vocal folds. The main aim was to determine the required extent of excision of the lesion in order to remove all pathological tissue to prevent possible recurrences by leaving potentially unstable areas. Previous studies of the epithelium in vocal fold lesions revealed thickening and destruction of basement membrane, reduplication of the lamina densa, near absence of normal hemidesmosomes and anchoring fibrils, abundant electro-dense vesicles at the basal cell pole (discharging through the cell membrane), abundant mitochondria, euchromatin and nucleolar prominence.12 The intercellular junctions were loose with widening of intercellular spaces, especially in basal and spinous layers. There were a high number of wide micro vessels in the sub epithelial space with an abnormal increase of layers of basement membrane-like material. Weibel-Palade bodies were demonstrated throughout the entire cytoplasm.13‒16
In this study of the junctional zone, the epithelium showed thickening and wide sub epithelial deposition of PAS positive material, which means excessive basement membrane material deposition that support the theory that the injury started at the BM and basal cell layer, leads to separation of the basal cell layer from the BM, on the ultra structural level, intercellular gapping was noted along with interrupted basal lamina and focal deposition of basement membrane like material, this intercellular gaping mostly caused by intercellular oedema that may be due to injury, this widening was more apparent with polyps than nodules.
The blood vessels of the lamina propria of the transitional zone showed disruption and fraying of the lamellated vascular basement membrane. The endothelial cells showed many thin microfilaments, marked thinning and shortening with widened intercellular junctions. Also, foci of detachment of the endothelial cells from the underlying basement membrane were noted. Some endothelial cells underwent apoptotic changes in the form of nuclear chromatin condensation, shrinking and fragmentation. These findings are nearly identical to those occurring in the lesion itself. Moreover changes in the blood vessels of the transitional zone seem to be even more pronounced than those in the lesions themselves.
Our findings suggest that the area of vascular and epithelial instability extends beyond the grossly visible pathology in the anteroposterior direction. This may be explained by the normal directional polarity of the structural elements of the vocal fold all running in the anteroposterior axis.17,18 This may imply the need for more distal and proximal excision of vocal fold mucosa to remove this unstable tissue which may induce further inflammatory reaction and induce recurrence of the disease.
None.
Author declares there are no conflicts of interest.
None.

©2017 Mostafa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.