Journal of
eISSN: 2379-6359


Case Report Volume 14 Issue 3
1Otolaryngology/Plastic Surgeon. Hospital Real San José Valle Real, México
2Plastic Surgeon, Hospital Real San José Valle Real, México
3Maxillofacial Surgeon. Unidad de Cirugía Maxilofacial, México
Correspondence: Julio Casas Ocando, Otolaryngology/Plastic Surgeon. Hospital Real San José Valle Real. Guadalajara. México, Tel +1(786)4633750
Received: August 11, 2022 | Published: October 17, 2022
Citation: Julio CO, Vázquez L, Jhoan AR. Radiofrequency treatment of labial hemangioma. J Otolaryngol ENT Res. 2022;14(3):80-84. DOI: 10.15406/joentr.2022.14.00511
Localized vascular lesions, hyperplastic or permanent dilation of vascular origin, not inflammatory or degenerative, are called angiomas. These hyperplasias may be of lymphatic vessels (lymphangiomas) or arterial vessels (hemangiomas), or both (hemolymphangiomas). Hemangioma is one of the most common benign tumors of vascular origin, present in childhood, located in the head and neck area (60%), the first location being the lip, followed by the tongue and palate; variable size, bright red or bluish red color, diagnosis by pressure changing color or by a puncture. A hemangioma can be flat, cavernous, papular, stellar, tuberous, and tumorous; characterized by three stages: endothelial cell proliferation, rapid growth, and spontaneous involution. Spontaneous resolution in 50% of cases and 90% of lesions before 9 years are solved. 20% of the cases are complicated, and the most frequent are ulcers with or without infection. Various procedures are described to solve it: surgery, cryosurgery, electrocoagulation, mechanical compression, systemic and intralesional corticotherapy, sclerotherapy, interferon alfa-2a, propranolol, selective embolization, laser therapy (DIODE, CO2, Nd: YAG) and radiotherapy; sometimes leaving as sequelae of treatment, scars. We present a case of a 25-year-old female patient with a labial hemangioma who had undergone two previous procedures, embolization, and arterial clip, without success. We use radiofrequency (coblation) since it is a method that allows achieving ablation, resection, coagulation, and hemostasis of blood vessels, with good results for the patient after its use, we carry out a systematic review of the hemangioma and the use of the coblator, in English and Spanish (Google Scholar, Cochrane, ResearchGate, Medline_Pubmed, LILACS, ScIELO, Medigraphic).
Keywords: hemangioma, benign head and neck tumor, coblator, vascular malformation, radiofrequency surgery.
Hemangioma turns out to be, the most frequent benign vascular disease in newborns during childhood and adolescence, but in some cases, it develops in adulthood;1 characterized by three stages: the disappearance of endothelial cells, rapid growth, and spontaneous involution.2 Presenting as a single lesion in the head and neck (60%),1,2 and in this area the most common are the lips, tongue, buccal mucosa, and palate, with spontaneous resolution in 50%,2,3 the 90% of the lesions are resolved before the first nine years of life, the difficult prognosis and, this does not depend on the size or location of the tumor, the behavior is expectant, before deciding to treat the hemangioma. It is three times more frequent in women than in men.2
It presents as, a single or lobulated lesion, small or large. Depending on the depth where they are found, it can be superficial (capillary hemangioma), deep (cavernous hemangioma) and mixed (venous or compound capillary hemangioma).2 Large and complicated hemangiomas, cause functional involvement of the lips (difficulty biting and feeding, and persistent salivation).1,4,5 The frequent complication is the ulcer with or without infection (20%),2 it occurs in a proliferation phase, in areas exposed to trauma, so it loses the normal lip anatomy. Management is controversial, hemangioma is a pathology of low incidence, hard to predict, and on which there are no randomized studies to evaluate the different therapeutic options, two treatment alternatives, expectant and medical or surgical therapy.6
In 1938, an article on the natural evolution of hemangiomas, was published in The Lancet by Lister and Camb, defending the principle of watchful waiting.7 However, detractors alleged that after reaching its maximum involution, around 25 % of hemangiomas present a significant deformity, which could be useful in small and slow-growing hemangiomas.6
Some authors consider monotherapeutic treatment with corticosteroids, to be the initial therapeutic action of choice for hemangiomas.1,8 Its effect was accidentally discovered more than 30 years ago in a child with thrombocytopenia, who presented a rapid involution of the hemangioma after treatment.9
The mechanism of action is unknown; however, it is believed that there is an inhibition of angiogenesis, an increase in mast cells and cytokines,10 and an increase in vasoconstriction and hormonal interference.6,10 Research conducted by Hasan et al.11 on the in vitro effects of various corticosteroids in a hemangioma model, determined that neither vascular endothelial growth factor (VEGF) nor fibroblast growth factor 2 (FGF-2) are important in inhibiting capillary growth corticosteroid-induced. Corticosteroids produce a significant decrease in the transcription of the interleukin (IL)-6 gene, a cytokine with proangiogenic activity. Corticosteroids increase sensitivity to vasoconstrictor substances in the microcirculation and prevent endothelial proliferation.12
There is no scientific basis for the use, type, dose, and frequency of corticosteroid administration.11 An initial dose of 2 to 3 mg/kg/day of prednisone or prednisolone is recommended, administered daily, in the morning, depending on the evolution, a dose reduction started for 4 to 8 weeks.8,13 Every patient receiving systemic corticosteroids should be evaluated monthly, or sooner if there are complications. One month after discontinuation of treatment, the patient is reassessed; if there is proliferation, the drug is resumed at 3-4 mg/kg/day, the patient after a week is evaluated, and if there is an improvement, the dose is decreased in the following four weeks.1,13 The intravenous and oral routes are equally effective, although they may be indicated at the onset of symptoms of heart failure, upper airway obstruction, or Kasabach-Merrit syndrome.
If the treatment is effective, the response appears in the first 2-3 weeks, if not, a progressive medication decrease should be carried out. According to some authors, in the first case, the dose should be maintained for at least one month,8 while others recommend a reduction to the minimum effective dose14 to avoid a rebound effect. Treatment should be maintained until complete involution of the hemangioma is achieved, which usually occurs after 8 to 10 months. In some centers rest periods of 2-4 weeks are prescribed after 4-6 weeks of treatment.14 Side effects of corticosteroids are usually avoided8 and are temporary. Appearance of cushingoid features (71%), personality changes (29%), gastrointestinal discomfort (21%) that can improve with taking ranitidine, oral or perineal fungal infection (6%), and sleep disturbances14 Less frequent effects have been described, such as osteoporosis, otitis media, transient steroid myopathy, pneumonia, sepsis14 and hypertension. Given the possibility of immunosuppression, it is recommended that varicella immunoglobulin be administered within the first 72 hours after exposure to the virus, to avoid disseminated infection6 and to explain the situation of immune suppression to family members13
Intralesional administration of corticosteroids is an effective treatment that avoids the adverse effects of systemic corticosteroids.1,8 They are indicated for well-localized cutaneous hemangiomas, mainly in the auricle, nasal tip, cheeks, eyelids, and lips. It is more effective to mix triamcinolone (40 mg/ml) with betamethasone sodium phosphate (6 mg/ml). In large lesions, it is suggested to initially combine 80 mg of triamcinolone and 16 mg of betamethasone, associating the rapid action of betamethasone with the prolonged action of triamcinolone.6 It is recommended to use fine needles, size 27-30G, aspirate after the puncture, perform multiple injections of 0.1 ml, and apply digital compression to avoid bruising. The treatment can be repeated at intervals of between 4 and 8 weeks, with a total of 3-4 sessions, or until the hemangioma returns. Complications are rare, the Azzolini group describes local adverse effects such as localized hypertrichosis, ecchymosis, and reversible skin atrophy, in 7.9%, as well as immediate local swelling that disappears in 24-48 hours, bruising, and necrosis.15
Clobetasol has been used as a topical corticosteroid, as well as betamethasone valerate, with inferior results to those found with Intralesional corticosteroids,16,17 therefore, it is not a possible alternative to Intralesional treatment. Successful treatments with propranolol for infantile hemangiomas also appear in medical publications, with good clinical tolerance, and without major side effects.1,4
Cytotoxic agents useful when administered in biologically benign diseases,1,6 such as: bleomycin, has been used Intralesional successfully, observing local sclerosis of endothelial cells, fibrosis, and spontaneous resolution of lesions after 2 or 3 treatments with 2 mg of bleomycin 30 days apart.18,19 Vincristine is effective in hemangiomas and vascular tumors associated with the Kasabach-Merritt phenomenon. At low doses, it acts as an angiogenesis inhibitor in murine model tumors. Cyclophosphamide, like vincristine, or low-dose cyclophosphamide, also acts as an angiogenesis inhibitor in murine model tumors. Pingyangmycin, chemically like bleomycin, with an antiangiogenic effect, is an antibiotic produced by Streptomyces pingyangensis, which alters the endothelium and has a sclerosing action,20 successfully used in hemangioma as monotherapy,21 or combined with surgery. Radiotherapy was used in hemangiomas1,8 and vascular malformations until the early 1960s, with aesthetically unacceptable results and long-term complications such as carcinogenic skin effects.22
The first identified endogenous anti-angiogenic regulators were the different interferons, the first treatment reference described for hemangiomas with interferon dates from 1989.23 In the treatment of hemangiomas, interferon alpha 2a1,6 and later beta 2 have been used successfully, appreciating that they stop the growth and favor a decrease in size, this treatment seems more effective when started at an early age,24 probably because a greater effect is achieved in the proliferative phase, like to what happens with corticosteroids but with greater potency than corticosteroids, the problem in its use is toxicity, limiting its use due to effects (fever, kidney failure, neurological toxicity)25 and high cost.1,8
The use of lasers began in 1962 (pulsed light, KTP, Nd-YAG, CO2, DIODE), useful in the initial stages of superficial lesions and the superficial portion of a deep hemangioma,8 as well as in ulcerated hemangiomas. The result, is minimal cell destruction and tissue inflammation, reducing postoperative pain, and limiting the depth of the lesion since they do not reach more than 2-3 mm.8,26-29
Intermittent and continuous pneumatic compression, its mechanism of action is unknown, but effective, used in hemangioma of the extremities, both in proliferative and involutional phase.30 Intermittent (pneumatic) and continuous pressure devices elastic bands31 and self-adhesive bands are used. Sclerotherapy, with sclerosing agents such as polidocanol 1%,29 or ethanolamine oleate,32 regression of the hemangioma, adverse effects such as skin necrosis and nerve paralysis33 are observed; arterial catheter embolization, also used as second-line treatment after systemic corticosteroids alone or associated with medical or surgical treatment,14 subject to the location, size, and evolution of the lesion. Complications include cerebrovascular accidents, distal embolization,33 and skin necrosis.14 Surgical treatment is performed in the absence of response to systemic treatment or for cosmetic reasons. Cryosurgery with liquid nitrogen1,3 is not a widely used technique. A variant has been described with a device that works at -32ºC and an early contact cryotherapy method that uses a constant temperature applicator that provides good cosmetic results.34 This last option is equally effective in superficial hemangiomas and more effective in deep or mixed ones.35 Procedure adverse effect: pain, skin atrophy, scar formation, and pigmentation changes.36
The flap surgery is also considered, as well as the use of electro cautery, as an option.1 However, despite all this arsenal of treatments, and the two previous failed treatment attempts, we thought about the possibility of using the COBLATOR II Surgery System, considering that it is a versatile device that allows ablation, resection, and coagulation of the soft tissues. The system dissolves tissue at the molecular level in a highly controlled manner with minimal thermal effect on surrounding tissue.37Low-frequency radiofrequency treatment (called ablation-controlled coblation) is a useful approach for the treatment of hemangioma. Its principle is the supply, locally through an electrode and a conductive medium, of low radiofrequency energy that induces local tissue destruction and creates secondary fibrosis, with little damage to the underlying tissue and rapid recovery.15 We think it is a useful alternative for the treatment of labial hemangioma, therefore, we carried out a systematic review of this pathology and the coblator use in it, both in English and Spanish (Google Scholar, Cochrane, ResearchGate, Medline_Pubmed, ScIELO, LILACS, Medigraphic), searching for published cases, author information, human case series, year of publication, age, sex, size of the lesion, and the number of cases. To our knowledge, this is the first case performed with the Coblator for the labial hemangioma treatment.
A 25-year-old female patient presented for consultation in January 2022, complaining of a violaceous tumor located in the right labial commissure, upper lip, and ipsilateral cheek mucosa, the tumor had been present since birth and had already been treated twice without success. The first treatment performed was selective arterial embolization and given no improvement, then they proposed surgery with an arterial clip, which did not have a favorable result either. On physical examination, a violaceous tumor is observed on the upper lip, right commissure of the mouth, with increased volume, soft and painless on palpation with a change in color on digital pressure, mouth opening without complications, and prolongation of the mucosal lesion of the right cheek, involving superficial and deep tissue
(Figure 1A-1C), based on clinical examination we came to a diagnosis of hemangioma.
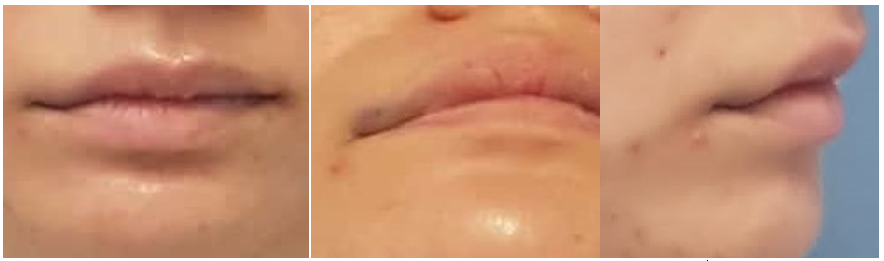
Figure 1A-1C Preoperative (frontal, base, and side view). Asymmetry of the upper lip and on the right corner of mouse. .
Under general anesthesia, we use the COBLATOR, due to its characteristics that allow us to perform ablation, resection, and coagulation of soft tissues, in addition to hemostasis of blood vessels (Figure 2).
We were careful with trans operative bleeding; we obtained a 90% reduction in tumor volume (Figure 3A-3C).
Six months follow-up has been carried out, up to now, the result is maintained (Figures 4&5).
Hemangiomas and vascular malformations are two distinct groups of vascular lesions. The term hemangioma encompasses a heterogeneous group of vascular lesions characterized by impaired growth and proliferation of endothelial cells. In contrast, vascular malformations are structural abnormalities of blood vessels without the proliferation of endothelial cells. The etiology and pathogenesis of hemangioma remain unknown, but common factors include childbearing age, gestational hypertension, and the baby's birth weight.38
There is controversy about how the management of hemangioma should be, since there is still no therapeutic guide based on evidence-based studies. It is uncommon to treat this pathology due to serious complications or aesthetic or functional alterations, and the expectant attitude while observing its natural evolution is usually sufficient, only 1% can be dangerous for the patient.1 10-15% of patients will need active treatment, of these the first treatment option, systemic corticosteroids,1 and is based on empirical recommendations, its management varies among doctors, few studies that support its real efficacy, and various conclusions,1 In hemangiomas in which corticosteroids have not been useful due to the characteristics of the tumor, systemic drugs such as vincristine or interferon can be used, but their use is limited by their serious side effects and their cost.1,8
Léaute-labréze et al.,39 in 2008, accidentally observed that by administering propranolol to nine children with severe or disfiguring capillary hemangiomas there was an immediate improvement in the color and size of the lesion without presenting adverse effects, four of these patients had not had a response to oral prednisolone.4
The regression of cutaneous hemangioma is explained through several mechanisms: 1.- vasoconstriction occurs, therefore there is a rapid lightening of the color and softening of the tumor, 2.- through the regulation of mitogenesis-activating protein kinase (RAF), there is less expression of the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) genes, which collaborates with the initiation of apoptosis of capillary endothelial cells.1
The doses of the different treatments, their increase, duration yet to be determined, not yet well established due to what will arise from the response to treatment, but there is consensus in maintaining it until the end of the elimination phase of the hemangioma (12 months of age), to avoid early recurrences.1,4 It has been described that labial hemangioma evolve more slowly, and are associated with feeding difficulties, persistent salivation, and difficulty in biting. In addition, they have a higher risk of leaving deformities, residual scars, or ulcerations.4 Treatment depends on the size, location, and evolution of the tumor. Systemic corticosteroid administration, Intralesional injection of sclerosing agents, electrolysis electrocoagulation, cryosurgery, laser therapy, cryotherapy, embolization, and surgical excision are some of the treatment modalities practiced for hemangioma.37,38 In this case, the patient is advised to perform surgical treatment with radiofrequency due to the lack of response to systemic treatment, demonstrating the use of COBLATOR to be an effective alternative, reducing swelling and bleeding during surgery.15
The treatment of hemangioma is still controversial since there is no treatment guide that guides how to treat the tumor according to its presentation and evolution, and evidence-based treatment, so we bring one more option as an alternative treatment. A detailed study of hemangiomas and their growth pattern is necessary to reduce unnecessary social embarrassment for the patient, and despite the different modalities recommended in the management of hemangiomas of the lip, surgery could be the main treatment if the necessary care measures are considered to perform it safely. The coblator (radiofrequency) as an alternative to hemangioma is a safe and effective procedure, to control and reduce the tumor by 90%, allowing adequate hemostasis, reducing the risk of bleeding and with a low inflammatory response. Long-term follow-up is necessary to assess the potential of this surgical tool. We leave open the possibility of a new session looking for 100% improvement
None.
None.
The authors deny public or private support.
The present article was carried out following the principles of the Declaration of Helsinki.

©2022 Julio, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.