Journal of
eISSN: 2379-6359


Case Report Volume 10 Issue 1
1Royal Marsden NHS Foundation Trust, Clinical Oncology, UK
2Royal Marsden Hospital, Histopathology, UK
3Royal Marsden NHS Foundation Trust, UK
Correspondence: Karla Lee, The Royal Marsden NHS University Trust, Clinical Oncology, UK, Tel 07445140403
Received: December 17, 2017 | Published: January 23, 2018
Citation: Lee KA, Newbold K, Thway K, Weller A (2018) Bone Marrow Failure in HPV-Associated Oropharyngeal Squamous Cell Carcinoma. J Otolaryngol ENT Res 10(1): 00302. DOI: 10.15406/joentr.2018.10.00302
A 56-year-old gentleman presented with bleeding from his mouth and gums. He also had a left neck lump and throat discomfort. He was found to have a p16+ oropharyngeal squamous cell carcinoma (OPSCC) of his left tonsil with ipsilateral level II and III nodal involvement. A PET scan revealed extremely diffuse metastatic disease involving all vertebral bodies, both humeri, femora and scapulae, pelvis, skull and other sites. He was anaemic and profoundly thrombocytopaenic as a result of his bony metastatic disease.
During the course of his disease his pain was difficult to control and he received regular packed red cells and platelets to support his failing bone marrow. A high suspicion of, and thorough investigation for, a second malignancy provided no answers. He received 2 cycles of dose-adjusted Paclitaxel chemotherapy but sadly did not respond to this and died 10 weeks following presentation due to bone marrow failure.
Keywords: oropharyngeal tumours, opscc, bone marrow, squamous cell carcinoma
OPSCC, oropharyngeal squamous cell carcinoma; HPV, human papilloma virus; SCC, squamous cell carcinoma
Oropharyngeal tumours account for more than 500,000 cases annually worldwide.1,2 The incidence rate in males exceeds 20 per 100,000 in regions of Hong Kong, the Indian subcontinent, parts of Europe and among African Americans in the United States.3 In Europe, there were approximately 250,000 cases and 63,500 deaths in 2012.4 A 2013 study designed to determine the prevalence of HPV-positive oropharyngeal cancer in an unselected UK population detected HPV in 55% of patients.5
This case describes an extremely unusual presentation of this relatively common malignancy. Our patient presented with complete failure of his bone marrow as a result of extremely diffuse metastases from his HPV-associated oropharyngeal squamous cell carcinoma (OPSCC). At the time of writing, there are no reports documenting disease of this extent at presentation of an OPSCC. This patient was thoroughly investigated for a second malignancy but none was found. Given recent reports associating P16 positivity and unusual metastatic behaviour of OPSCC’s,6‒12 this very unusual case serves to strengthen this association.
A 56-year-old gentleman presented with a 6-week history of gum bleeding and easy bruising. He also had a palpable left neck lump, mild throat discomfort and back pain. He had a history of hypertension and mild gastritis. He was a current smoker of 15 cigarettes per day with a 40 pack year history and an alcohol intake of 20units per week. He had no significant family history. On examination he had a left-sided 10cm fixed lymph node mass extending from level II to III. Flexible nasendoscopy confirmed an obvious tumour extending from the left tonsil to the tongue base. The vallecular appeared free and the vocal cords were mobile.
Investigations
Neck ultrasound revealed an enlarged pathologic non-vascular level II cervical lymph node. Biopsy of both this and the tonsillar lesion revealed poorly differentiated squamous cell carcinoma. P16 immunostain was strongly and diffusely positive (Figure 1).

Figure 1 H&E showing almost complete replacement of marrow by squamous cell carcinoma. The tumour is strongly and diffusely p16 positive.
MRI Neck revealed a 7cm lymph node mass at level II. This involved the left tongue base, left lingual tonsil, palatine recess and palatine tonsil. Extensive abnormal signal intensity suggestive of metastatic disease was noted in the bones including the vertebral bodies, upper ribs and clavicles.
A whole-body PET-CT scan was performed. This revealed FDG avidity on the left side of the neck with further abnormal foci noted within the left tonsillar bed, lateral left side of the tongue base and lower cervical node chain (Figure 2). It also showed tracer uptake of the skull vault, skull base, mandible, all of the vertebral bodies, both humeri, both scapulae, multiple ribs, sternum, pelvis and both proximal femora. At these sites there was evidence of mixed lytic and sclerotic disease. No encroachment of the spinal cord was seen. Three small FDG avid intrahepatic lesions were also noted and there was a small FDG avid lower right-sided para-aortic node also (Figure 3). Spleen, kidneys, adrenals, bowel and prostate were noted to be normal. Laboratory panel showed a haemoglobin of 81g/dL, white cell count of 7.2, platelets of 5 and an LDH of 1057. Other markers, including calcium, were within the normal range. A blood film showed a leucoerythroblastic picture with normocytic, normochromic red cells, some polychromatic cells, thrombocytopaenia, a left neutrophil shift, myelocytes and occasional blast cells. Lymphocytes were small and mature.
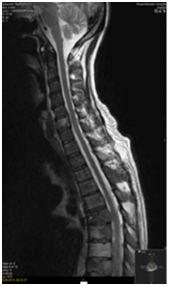
Figure 2 18FDG PET confirming the head and neck primary plus multiple spinal, Sternal, Pelvic, Humeral and Extra-osseous metastases (including liver).
A bone marrow trephine revealed marrow almost entirely effaced by metastatic carcinoma. The tumour was composed of cohesive nests/islands of tumour in abundant desmoplastic stroma. Immunochemistry showed the carcinoma was positive for p40, p63 and Cytokeratin 5/6 and was entirely consistent with metastatic squamous cell carcinoma. It was also p16 positive (Figure 4). The morphology was also in line with this diagnosis. PSA was 0.3 and CEA, CA19-9, AFP were negative. HIV, Hepatitis B and C, B12 and folate were all normal/negative.

Figure 4 Immunohistochemistry for p16 shows diffuse and strong nuclear and cytoplasmic expression in all neoplastic cells of bone marrow.
Differential diagnosis
Given the degree of diffuse bony disease and bone marrow failure we were extremely suspicious that this gentleman had a second metastatic primary cancer. A metastatic prostate adenocarcinoma would have been the most likely cause for such a presentation but we gave consideration to all tumours with a propensity to metastasise to bone. PET-CT showed avidity at none of these sites. A thorough tumour marker screen was also negative. We also considered a haematological malignancy but the trephine was not consistent with this. Fanconi’s anaemia was given consideration but this gentleman had none of the phenotypic traits, nor the longstanding history of anaemia associated with this condition.
This gentleman was profoundly marrow suppressed. He bled from both his mouth and nose for the months leading up to his death. Tranexamic acid was taken daily and he was transfused platelets and packed red cells on a regular basis. Consideration was given to delivering radiotherapy to his oropharynx for control of bleeding but this was not required. His pain was difficult to control and we struggled to achieve a steady state. Much input from palliative care services was required.
An MRI Whole spine was performed following his PET-CT given the extent of vertebral disease. Abuttment but not compression of the cord at the level of T6 was seen (Figure 5). Two weeks following this patient developed some neurological symptoms attributable to progression at this site. Multiple wedge fractures and imminent compression of the cord was seen. A neurosurgical opinion was sought and this patient was deemed not to be for surgical intervention. Twenty Gray in 5 fractions of palliative radiotherapy was delivered to T5, T6 and T7 vertebrae.

Figure 5 Expansile metastases including at T6 narrowing the spinal canal and indenting the cord on
MRI spine.
Following a lot of multidisciplinary discussion and acceptance that the diffuse bony disease was caused by the primary oropharyngeal tumour, the decision was made to offer palliative chemotherapy. The patient was given 2 cycles of Paclitaxel at 40mg/m2 which were reasonably well tolerated.
Outcome and follow-up
Unfortunately palliative chemotherapy failed to halt this gentleman’s bone marrow progression and he died within 10weeks of diagnosis.
Over the last few decades it has become clear that human papilloma virus (HPV) is responsible for a marked increase in the incidence of OPSCC.13 Greater than 50% of all OPSCCs are associated with high risk HPV and stain positive (+) for P16, a surrogate marker for HPV.14,15 Oropharyngeal cancer associated with HPV appears to have a distinct molecular, epidemiological and clinical behaviour. Multiple retrospective and prospective studies have shown that patients with P16+ oropharyngeal tumours have better outcomes than those staining negative (-) for this immune-marker, despite often having a higher nodal burden at presentation.14,16,17
Loco-regional control rates are generally excellent amongst p16+ tumours and poor outcomes are mainly attributable to distant metastases.18 Distant spread is uncommon and usually occurs late in the disease course, typically in the setting of advanced localregional disease.19‒21 The incidence of distant metastases in head and neck squamous cell carcinoma (SCC) is relatively small in comparison to other malignancies.22 OPSCC metastasing to bone is also uncommon, with pulmonary metastases being seen more frequently.
Despite improved survival amongst p16+ patients, peculiar and unusual patterns of distant metastases have been reported in recent years, often involving bone-only sites of metastases.8,12,23 These HPV-related cancers have an epidemiologic, demographic and clinical profile that deviates from the profile of conventional non-HPV-related OPSCC.11,6,10,7 Huang et al. report distant metastases rates are similar but that the natural course of HPV+ distant metastases differs from that of HPV- patients and often demonstrates a "disseminating" phenotype.9 Muller et al.,12 report on 4 cases of atypical metastases of p16+ OPSCC; all 4 presented with only locoregional disease but developed bony +/- other sites of metastases following chemoradiotherapy. They remarked on the rapidity of development of distant disease. In a recent retrospective cohort study, Huang et al. described unusual behavior in P16+ OPSCC patients treated with radiotherapy. They detailed distant metastatic spread to multiple organs and unusual bony sites.8
A study by Sinha et al. set out to characterise the patterns and clinical outcomes of distant metastases in p16+ OPSCC and reported that while the “disseminating” phenotype was almost twice more common than that of the p16- group, the difference was not statistically significant.18 Rozevick et al.,24 report that while HPV positivity is generally felt to be a favorable prognostic indicator, it does not safeguard from spread to the brain. To our knowledge, only these aforementioned papers have remarked on unusual metastatic behavior of P16+ tumours. At the time of writing, there are no reports in the literature of patients presenting with marrow failure, or indeed of anything close to the bony disease burden seen in our patient. Knowledge of the atypical behaviour occasionally exhibited by HPV-associated OPSCCs should cause us to consider whether patient work-up and follow-up should differ between p16 positive and negative patients. Publication of further case reports and series is needed.
None.
Author declares there are no conflicts of interest.
None.

©2018 Lee, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.