Journal of
eISSN: 2377-4282


Research Article Volume 4 Issue 2
1Department of Food Science, Cairo University, Egypt
2Nanotechnology & Advanced Materials Central Lab, Agriculture Research Centre, Egypt
Correspondence: Taher A Salaheldin, Nanotechnology & Advanced Materials Central Lab, Agriculture Research Center, Egypt
Received: July 11, 2016 | Published: August 31, 2016
Citation: Raya SDHA, Hassan MI, Farroh KY, Hashim SA, Salaheldin TA (2016) Zinc Oxide Nanoparticles Fortified Biscuits as a Nutritional Supplement for Zinc Deficient Rats. J Nanomed Res 4(2): 00081. DOI: 10.15406/jnmr.2016.04.00081
Zinc deficiency is a global nutritional disorder affecting majority of healthy people rather than those suffering from chronic diseases, affects about one third of people around the world and thereby causing clinical manifestations. This work represents new modality to increase the intestinal absorptivity and bioavailability of zinc from the diet by replacing traditional bulk form by zinc nanoparticles to be used in preparations of zinc fortified biscuits as a nutritional supplement for zinc deficiency disease in rats. Zinc oxide nanoparticles of 25 ± 5 nm sizes were synthesized by co-precipitation approach and characterized by Transmission Electron Microscope and X-Ray Diffraction. Zinc deficient rats model was performed by nourishing on zinc deficient diet for five weeks to acquire zinc deficiency. Three levels nano zinc oxide Fortified Biscuits (13.5 ppm, 27 ppm and 54 ppm) were prepared to be compared with bulk form of zinc oxide. Results showed that, rapid enhancement of body growth rate, appetite and hair growth was recorded for nano zinc treated groups that might be attributed to the increased intestinal absorptivity and bioavailability of the nano scaled zinc oxide as a result of its ultra-small sizes compared to the regular bulky zinc oxide form. The toxicological evaluation of the applied nano zinc oxide concentrations didn’t cause any apparent toxicity and the lethal dose (LD50) cannot be established within the applied doses, where no mortality during the designed experiment period. Histopathological examination support the capability of the nano zinc oxide formulation to replace the traditional bulk forms of oral zinc supplements for rapid and efficient recovery with no significant histopathological abnormalities in Liver, Kidneys and Testis. The present work recommend dose of 13.5 ppm nano zinc fortified biscuits for managing mild zinc deficiency and the dose of 27 ppm for more sever conditions.
Keywords:Nano zinc oxide, Nanofortification, Zinc deficiency, Nanofood
Zinc deficiency is one of the most abundant global nutritional related health problems involving nearly about one-third of world population, the great majority resident in the developing country and the risk is highest between very poor population where animal food products, the best sources of zinc, are limited.1 The mean reason of zinc deficiency is largely related to inadequate intake or malabsorption of zinc from the diet. The distinction between intake and malabsorption is important: high levels of dietary inhibitors (such as fibre and phytates) may suppress the absorption of zinc, even though high intake of zinc. For this reason, zinc requirements for dietary intake are adjusted upward for populations in which food products of animal origin (such as meat, fish and dairy products) are limited, and in which plant sources of zinc are high in phytates.2,3 The health consequences of sever zinc deficiency are very serious characterized by short stature, hypogonadism, impaired immune function, skin disorders, cognitive dysfunction, anorexia and reduced physical work performance at all ages.4-6 There is correlation between zinc deficiency and growth retardation as a result of reduced appetite that can be reflected in low food intake and low body weight, although zinc deficiency did not affect relative organ body weights.7 Zinc is a component of many enzymes that could affect the metabolic growth in infancy and childhood, sexual Maturation and immunity.8 Zinc-supplemented pre-school children have a 9% reduction in all-cause mortality and an 18% reduction in deaths in children 12-59 months of age. There were also statistically significant reductions in diarrhea (13%) and pneumonia (19%) incidence.9,10
Oral Zinc supplementation is still the major route for management of zinc deficiency although it is not well tolerated by all patients even with high doses because of the intestinal zinc absorption is not always adequate. Food fortification is considered to be the most cost-effective strategy for combating long term nutritional Zinc deficiency in population groups that buy one or more commonly eaten food items. It is an attractive strategy because consumer compliance is ensured; it can be both inexpensive to initiate and sustainable.11 Frequently used forms of zinc in supplements include zinc sulfate, zinc oxide, zinc acetate and zinc gluconate.12 Recently, the use of nano scale micronutrients fortification have great attention due to their ultra-small size which facilitate the intestinal absorption such as nano iron fortification for management of iron deficiency anemia.13,14 and zinc nanoparticles formulations were used to replace the frequently zinc salt with great efficiency and lower side effects.15,16 The present work represents new modality for management zinc deficiency by increasing the intestinal absorptivity of Zinc through Synthesis Nano Zinc Oxide (ZONPs) to prepare zinc fortified biscuits and to compare zinc oxide in bulk form, aiming to produce cost effective, tasty and easy to produced zinc food supplements. The study was performed on rats and the efficiency was conducted from nutritional and toxicological point of view.
Synthesis of zinc oxide nanoparticles
25 ± 5 nm Zinc Oxide Nanoparticles (ZONPs) were synthesized by co-precipitation Method.17 with little modifications. In brief, 2.97gm zinc nitrate hexahydrates (98% Sigma-Aldrich, USA) dissolved in 100 ml deionized water (Milli-Q, Millipore, USA). 0.48 gm sodium hydroxide (NaOH, 98%, Sigma-Aldrich, USA) was dissolved in 60 ml deionized water, then was added to zinc nitrate solution drop wise under vigorous stirring for 12 h. The precipitate zinc oxide nanoparticles was dried at 100 °C and grinded to fine powder using agate mortar to be calcined at 900 oC for 2 h. The obtained ZONPs were characterized by Transmission Electron Microscope (TEM, Tecnai G20, FEI, Netherlands) and X-ray Diffraction (XRD, X’Pert Pro, PanAlytical, Netherlands). All the Preparation and characterization processes were conducted at Nanotechnology and Advanced Materials Central Lab (NAMCL), Agriculture Research Center, Egypt.
Preparation of nano zinc fortified biscuits
Nano zinc fortified biscuits with three ZONPs concentration levels (13.5 ppm, 27 ppm and 54 ppm) were prepared according to cookie method 10-52-02 (AACC 2010).14,18 with slight modifications. In brief, the selected concentrations of ZONPs were dissolved in 27 ml deionized water to be used during blending process to ensure proper mixing of the zinc nanoparticles with the flour. Dry ingredients (sugar, shortening, sodium carbonate and sodium chloride) and eggs were blended with the flour and dough thoroughly kneaded. Figure 1 illustrates the composition of ZONPs fortified biscuit. The dough was then placed on a cutting board, rolled out until uniform thickness and textures were obtained. Biscuit cutter was used to cut the sheet of rolled dough into desired shapes and sizes, then baked in oven at 220 °C for about 15 min. the cooked biscuit was allowed to cool at room temperature, packed and store according to Kure et al.19 To achieve homogeneous flour and to ensure the level of fortification claimed, samples of the fortified flour were collected during blending and assayed for zinc concentration by Inductively Coupled Plasma Optical Emission Spectrometry, ICP-OES, technique (Thermo Scientific iCAP 7000, USA). The measurement was carried out until the concentration of zinc was similar in the sample taken from various section of the dough. Reference fortified biscuit was prepared using bulk zinc oxide form (13.5 ppm) instead the nano form.
Zinc deficient animal model
Three weeks old Albino male rats, weighing 60 -70 g, were accommodated and adapted at animal house unit, Faculty of Agriculture, Cairo University, Egypt. The experimental and accommodation procedures were conformed according to the NIH guidelines for animal health and accommodation.20 The laboratory animal facility is maintained under a 12 hour light/dark cycle at a temperature (22 ± 2) °C, and relative humidity of 30-40 %. The animals were divided into six groups of six rats each, namely as a following;
All groups except normal control negative group were fed Zn deficient basal diet for five weeks until the symptoms of Zinc deficiency appeared (e.g. loss of appetite, hair loss, and decrease in food intake and body weight) and serum Zn concentration were assessed and compared to the normal control negative group. The zinc free basal diet was replaced with the nano zinc fortified biscuits for the nano zinc treated groups (13.5 ppm, 27 ppm and 54 ppm) and reference group (13.5 ppm bulk zinc oxide) for four week. The composition of the basal diet was formed of casein 10 % supplemented with L-methionine 0.22 % of the total solids of diet. The diet contained 10 % sucrose, 69 % maize starch, cellulose 5 %, maize oil 5 %, mineral blend 4.8 %, vitamin 0.8 %, and choline chloride 0.2 %.14,21
Feed intake and body weight gain
Anorexia, Loss of appetite is one of the most physical symptoms of zinc deficiency resulting in low feed intake and consequently decreased body weight. Feed intake was measured in form of the amount of diet taken by the animal per day. While body weight gain was calculated once weekly, weighting each animal during the deficiency (five weeks) and recovery periods (four weeks).
Serum biochemical analysis
Zinc level in serum was assessed where blood samples were collected at the end of designated periods before and after treatment (three time points of analysis in total). Inductively Coupled Plasma Optical Emission Spectrometry, ICP-OES, technique (Thermo Scientific iCAP 7000, USA) was used to measure zinc level in animal serum.
Histopathological examination
Rats were euthanized at the end of experiment. Tissues of Liver, kidneys and testis were dissected and immediately fixed in 10 % formalin solution at room temperature. Autopsy samples (5-4µ tissue sections) were fixed in 10% formalin saline for twelve hours. Serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used. Specimens were cleared in xylene embedded in paraffin at 56 degree in hot air oven for twenty four hours. Paraffin bees wax tissue blocks were prepared for sectioning at 4 micron thickness by slide microtome. The obtained tissue sections were collected on glass slides, deparaffinized and stained by Hematoxylin and eosin (H & E) and subsequently processed for histopathological examination under upright light microscope (Leica DM5000, Germany).22
Statistical analysis
All measurements were carried out in triplicate and the results were expressed as mean ± S.D. Analysis of variance was performed using SPSS statistical package program (ver.18), with significance level 0.05.
Preparation and characterization of ZONPs
Nearly mono dispersed Zinc oxide nanoparticles, ZONPs, were well prepared by co-precipitation method. High Resolution Transmission Electron Microscopic (HR-TEM) image showed well-formed nearly spherical shaped particles with average size of 25 ± 5 nm as illustrated in Figure 3a. For phase analysis, X-Ray Diffraction (XRD) pattern of the as-prepared Zinc oxide nanoparticles is shown in Figure 3b. Characteristic pattern peaks of ZnO are observed at 2θ values of 32,35,37,47.5, 56.5, 63, 66, 68, 69, 72.5 and 77 in the pattern consistent with the hexagonal phase structure ZnO (ICCD #. 01-079-5604). The estimated crystal size using Scherer’s equation was 25 nm; the obtained results were in accordance with the previously published literatures.17,23
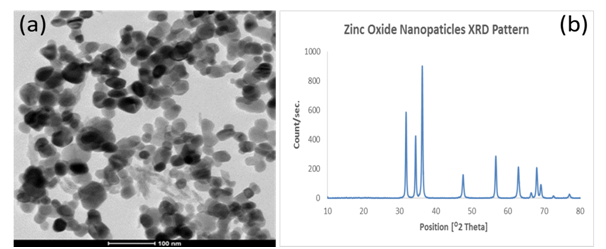
Figure 3 Characterization of the as prepared Zinc oxide nanoparticles. a: Transmission Electron Microscopic image, b: X-Ray Diffraction phase Pattern.
Feed intake and body weight gain
Anorexia, loss of appetite, is one of the most physical symptoms of zinc deficiency resulting in low feed intake and consequently decreased body weight. This observation was recorded during the conduction of the animal experiment. Results showed that during the zinc deficiency feeding period, five weeks, there was a significant decrease (P < 0.05) in daily feed intake by rat (diet in gram/ day/ rat) for all groups and control negative group was taken for comparison as shown in Table 1. While during recovery period, four weeks, there is a significant increase (P < 0.05) in the mount of feed intake/ rat/ day, reflecting increased appetite and reduced anorexia.
|
Animal group |
Deficiency Period |
Recovery Period |
|||||||
|
1st Week |
2nd Week |
3rd Week |
4th Week |
5th Week |
1st Week |
2nd Week |
3rd Week |
4th Week |
|
|
Control Negative |
19.4± 0.27a |
19.6± 0.29a |
19.3 ± 0.53a |
19.5 ±0.33a |
19.5 ±0.36a |
19.6 ± 0.32a |
19.5 ± 0.41a |
19.2 ±0.63a |
19.3 ±0.11a |
|
Control Positive |
17.3± 0.57a |
15.5± 0.44b |
13.9 ±0.64b |
12.6 ±0.69c |
10.8 ±0.48c |
8.9 ±0.76cd |
8.2 ±0.54d |
8.0 ±0.87d |
7.8 ±0.79d |
|
Nano Zinc (13.5 ppm) |
18.1± 0.65a |
16.2± 0.88 b |
14.5 ±0.63bc |
12.4 ±0.12c |
10.5 ±0.18c |
11.8±0.36cb |
13.7 ±0.58b |
16.9 ±0.67a |
19.8 ±0.64a |
|
Nano Zinc (27 ppm) |
17.7± 0.61a |
15.9± 0.45 b |
13.9 ±0.74bc |
12.1 ±0.11c |
10.2 ±0.33c |
12.4±0.21b |
14.8 ±0.65b |
17.8 ±0.57a |
20. 3±0.72a |
|
Nano Zinc (54 ppm) |
17.9± 0.17a |
14.9± 0.94 b |
13.1 ±0.24bc |
11.9 ±0.45c |
10.0 ±0.20c |
13.0±0.61b |
15.3±0.51b |
18.2 ±0.67a |
22.7 ±0.81a |
|
Bulk Zinc (13.5 ppm) |
17.0± 0.28a |
15.7± 0.26 b |
13.6 ±0.55b |
12.3 ±0.21bc |
10.3 ±0.27c |
10.7±0.27c |
12.1 ±0.21cb |
13.2 ±0.34b |
14.3 ±0.43b |
Table 1 Feed intake (diet in gram/ day/ rat), (mean ± SD)
Similar observations were recorded for body weight gain; weight losses (during the first five weeks) recorded more than 60% loss of its original body weight at the beginning of the experiment as a result of nutritional zinc deficiency and loss of appetite. Rapid recovery was recorded during the recovery period, four week nourishing on Nano Zinc diet, where remarked body weight gain with respect to the zinc nanoparticles concentration compared to reference group (bulk form zinc oxide), as shown in Table 2. The obtained result are in accordance with previously published data [24] and reveals that nano scaled zinc oxide fortified biscuits have higher metabolic rate compared to the traditional bulk form. That might be attributed to the ultra- small size of ZONPs which increases the surface area and rapidly facilitate the intestinal absorption and hence higher its bioavailability.
|
Animal group |
Body Weight During Deficiency Period(g) |
Body Weight During Recovery Period(g) |
|||||||
|
1st Week |
2ndWeek |
3rd Week |
4thWeek |
5th Week |
1st Week |
2nd Week |
3rd Week |
4thWeek |
|
|
Control Negative |
62.1 ± 1.8a |
67.2 ± 2.26 a |
73.4 ± 2.44a |
78.8 ±3.25 a |
84.2 ±3.32a |
88.5 ± 4.4a |
91.6 ± 4.77a |
96.3 ±4.87a |
103 ±5.31a |
|
Control Positive |
61.4 ±2.11 a |
57.2 ±1.88b |
54 ± 2.21bc |
51 ± 2.19c |
47.3 ± 2.35cd |
44.6 ±2.77c |
42.6 ±2.52c |
40.1 ± .87df |
38.8 ±3.89f |
|
Nano Zinc (13.5 ppm) |
60.6 ± 1.83 a |
57.3± 2.88 b |
53.4 ±2.53bc |
47.4 ±2.92c |
45.6 ±2.68cd |
49.3 ±2.22cd |
55.2 ±2.13cb |
63.8 ±3.67b |
70.7 ±2.64b |
|
Nano Zinc (27 ppm) |
61.3 ± 2.2 a |
58 ± 1.66 b |
55.41 ± 2.1b |
50.1 ± 2 .32bc |
45.77 ±2.5cb |
51.2 ±2.81c |
58.38 ±2.6b |
65.2 ±2.44b |
73.4 ±3.12b |
|
Nano Zinc (54 ppm) |
62.3 ± 2.13 a |
59.8 ± 2.91b |
55. 7 ±2.55b |
51.6 ± 2.26bc |
44.3 ± 3.16c |
52.6 ± 2.24bc |
60.5 ± 2.34b |
68.7 ± 2.77b |
77.9 ± 3.61b |
|
Bulk Zinc (13.5 ppm) |
61.7 ± 3.14 a |
58.2 ± 2.54 b |
53.1 ± 2.33b |
50.2 ± 2.47b |
45.9 ± 3.02c |
47.5 ± 2.12c |
52.5 ± 2.56cb |
58.1 ± 2.61b |
62.3 ± 3.27b |
Table 2 Body weight and weight gain, (mean ± SD)
Dermatological effects
Loss of hair is one of the dermatological side effects resulting from zinc deficiency disease. Zinc deficiency actually can lead to deterioration of the protein structure that makes up the hair follicle and acts as co-enzyme for many metabolic processes. This weakening of follicles can in turn cause hair shedding and hair loss. The experimental data showed sever hair loss in zinc deficient animal groups during period of feeding on zinc deficient diet. Oral administration of nano zinc fortified biscuits sustain the zinc deficiency in a very short time, where hair recovery and start of new hair growth was observed after two weeks of treatment as shown in Figure 4. The results suggest the future use of nano formulated zinc formula in management and treatment of hair loss resulting from nutritional zinc deficiency.
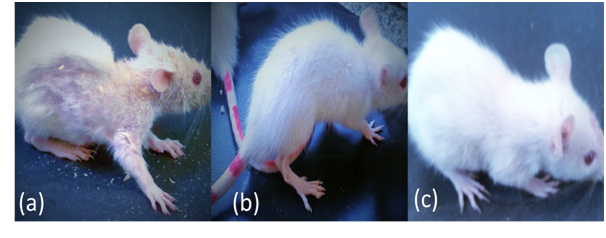
Figure 4 Photos of dermatological effect of zinc deficiency. (a) Hair loss of zinc deficient rat, (b) Hair recovery of nano zinc oxide nourished groups, (c) Rat of normal control negative group.
Effect on organs growth rate
To evaluate the effect of oral administration of zinc fortified biscuits on the growth of organs, liver and kidneys of all treated groups were weighted at the end of the designed period and compared to those of the normal control negative group. It was postulated that the growth of the bosy organs affected by zinc deficiency exactly as the body weight. Results were against such assumption, although zinc deficiency decrease body weight but not affect the internal organs weight particularly for liver and kidneys. Table 3 showed that there were no significant differences in weights of liver or kidneys of all zinc deficient groups compared to the normal control, except for the liver of nano zinc group at higher concentration, 54 ppm, showed slight increase (4.4 ± 0.61 g) compared to the normal control negative group (4.2 ± 0.24). The obtained results were agreed with previously published work revealed that, zinc deficiency has minor effect on the body organs growth rate.7
|
Animal Group |
Organ’s Weight (g) |
|
|
Liver |
Kidney |
|
|
Control negative |
4.2 ± 0.24 |
1.24 ± 0.19 |
|
Control positive |
4.1 ± 0.18 |
1.25 ± 0.21 |
|
Nano Zinc (13.5 ppm) |
4.1 ± 0.56 |
1.24 ± 0.19 |
|
Nano Zinc (27 ppm) |
4.2 ± 0.42 |
1.12 ± 0.20 |
|
Nano Zinc (54 ppm) |
4.4 ± 0.61 |
1.18 ± 0.32 |
|
Bulk Zinc (13.5 ppm) |
4.1 ± 0.23 |
1.16 ± 0.26 |
Table 3 Organ’s weight (mean ± SD)
Effect on Zn serum level
Zinc deficiency was performed by feeding the experimental rats on zinc free basal diet for five weeks until the serum zinc concentration decreased compared to the control negative group. Treated groups were divided into three levels of nano zinc oxide fortified biscuits, (13.5 ppm, 27 ppm and 54 ppm) and one level of bulk zinc oxide fortified biscuits (13.5 ppm) in addition to untreated control groups, control negative and control positive, nourished zinc rich basal diet and Zinc free diet respectively. Replacement of the zinc deficient diet by nano zinc oxide fortified biscuits for four weeks sustain the zinc serum level to be similar to or higher than reference values of the control negative group as shown in Table 4. The nano zinc formula increased serum zinc concentration with rapid and more efficient way rather than the bulk zinc form. In addition to that the magnitude of the serum zinc concentration is dose dependent, where serum zinc of animal group of 13.5 ppm nano zinc was 164.81 ± 2.34 µg/dI while group of 54 ppm nano zinc was 194.55 ± 3.66 µg/dI, which are higher than the normal control negative group, 160.56 ± 2.46 µg/dI. These observation was similar to the work done on Markhoz goat kids, where Zn supplementation elevate the plasma Zn concentration but does not affect growth performance and composition of the other blood minerals.25
|
Animal Group |
Serum Zinc Level (µg/dI) |
|
|
Deficiency Period |
Recovery Period |
|
|
Control negative |
150.96 ± 3.35 |
160.56 ± 2.46 |
|
Control positive |
110.5 ± 2.91 |
90.07 ± 2.47 |
|
Nano Zinc (13.5 ppm) |
115.30 ± 2.89 |
164.81 ± 2.34 |
|
Nano Zinc (27 ppm) |
123.15 ± 2.84 |
179.85 ± 2.03 |
|
Nano Zinc (54 ppm) |
131.25 ± 2.87 |
194.55 ± 3.66 |
|
Bulk Zinc (13.5 pm) |
128.15 ± 2.95 |
140.54 ± 2.86 |
Table 4 Serum Zinc concentration, (mean ± SD)
Toxicological evaluation
Although the nutritional efficiency of the nano Zinc fortified biscuits for management and control of zinc deficiency disease was successfully evaluated, but the potential toxicity must be evaluated at the applied concentrations. One of the main toxicological evaluation techniques is the histopathological microscopic examination of tissue organs to estimate the effect of the tested materials at tissue level. At the end of the designed experiment period, rats of all groups were euthanized and the internal organs were anatomized, the selected organs were Liver, Kidneys, and testis, Figure 5 summarized the observations of the histopathological microscopic examinations of all animal groups. Liver of the negative control group showed no abnormal histopathological alteration with normal of central vein and surrounding hepatocytes. While the effect of zinc deficiency was clearly observed in the positive control group where hyperplasia of epithelial lining bile duct, fibrosis of portal triad and portal infiltration with inflammatory cells were recorded. All nano zinc oxide nourished groups showed no histopathological changes except Kupffer cells activation that may attributed to the immunological response. While liver of the group nourished bulk form of zinc oxide fortified biscuits showed congestion of central vein and degeneration of hepatocytes. Kidneys of all treated animal groups showed the normal histological structure of the glomeruli and renal tubules except the 54 ppm nano Zinc oxide group showed mild congestion of intertubular blood vessels.26Control positive group (feed Zinc Free diet) showed the major zinc deficiency renal pathological abnormalities such as congestion of glomerular tuft, vacuolation of renal tubular epithelium and presence of proteinaceous materials in the lumen of renal tubules. Histopathological examination of the Testis of nano zinc oxide fortified biscuits groups showed no histopathological changes and normal seminiferous tubules, while the group nourished bulk zinc oxide diet showed slight degeneration of spermatogoneal cells lining seminiferous tubule. The control positive group showed degeneration and necrosis of spermatogoneal cells lining seminiferous tubules as a result of the nutritional zinc deficiency. From the toxicological point of view, feeding on nano zinc oxide fortified biscuits with the applied concentrations showed no significant or critical histopathological alteration with the examined organs (Liver, Kidneys and Testis) compared to those of the normal control negative groups. The obtained results support the capability of the nano zinc oxide formulation to replace the bulk forms of oral zinc supplements for rapid and efficient management of zinc deficiency disease or at least as a protective and preventive route.
Nano zinc oxide fortified biscuits was applied as a food supplement to manage and control nutritional zinc deficient disease in rats. The nutritional evaluation of zinc deficient rats nourished nano zinc fortified biscuits revealed that, rapid enhancement of body growth rate, appetite and hair growth was recorded that might be attributed to the increased intestinal absorptivity and bioavailability of the nano scaled zinc oxide as a result of its ultra-small sizes compared to the regular bulky zinc oxide form. Another achievement for nano zinc formula over the bulk form was the rapid recovery with non-significant side effects. The toxicological evaluation of the applied nano zinc oxide concentrations (13.5 ppm, 27 ppm and 54 ppm) didn’t cause any apparent toxicity and the lethal dose (LD50) cannot be established within the applied doses, where no mortality during the designed experiment period. Histopathological examination support the capability of the nano zinc oxide formulation to replace the traditional bulk forms of oral zinc supplements for rapid and efficient recovery with no significant histopathological abnormalities within the examined organs (Liver, Kidneys and Testis) compared to the normal control negative animal groups. The present work recommend dose of 13.5 ppm nano zinc fortified biscuits for managing mild zinc deficiency and the dose of 27 ppm for more sever conditions. But still there is a great need for extra studies and toxicological evaluations before marketing.
None.
None.

©2016 Raya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.