Journal of
eISSN: 2377-4282


Short Communication Volume 1 Issue 2
Department of Veterinary and Primate Health, Global Primate Network, Nepal
Correspondence: Tirth Raj Ghimire, Department of Veterinary and Primate Health, Global Primate Network, Kathmandu, Nepal, Tel 00 97714334784
Received: September 17, 2014 | Published: October 25, 2014
Citation: Ghimire TR (2014) Visualizing Particulate Antigen Targeting to Dendritic Cells (DCs) In vitro. J Nanomed Res 1(2): 00007. DOI: 10.15406/jnmr.2014.01.00007
Dendritic cells (DCs) are the unique antigen presenting cells (APCs) that can bridge innate and adaptive immune responses. Their roles in antigen uptake, processing, and presentation have been upgraded by various tools and techniques of biomedical research. The current article describes how the targeting of particulates by bone marrow-derived DCs (BMDCs) can be observed in vitro. This issue has been addressed by using flow cytometry and confocal microscopy. Elaborating these mechanisms will help understand the targeting activities of DCs in vitro and in vivo.
Keywords: Antigen uptake, Micro particle, Particulate, Phagocytosis, Pinocytosis, Presentation, targeting
DCs are central to the induction of adaptive immunity through their unique ability to activate naive T cells.1 To initiate an adaptive immune response, a number of signals are required in naive T cells. Among these signals, signal 1 is the cognate signal provided by peptide: major histocompatibility II (p:MHCII) complexes expressed on DC.2,3 To provide this signal, DCs internalize an exogenous protein antigen, process it into peptides, load these onto MHC molecules and export these complexes on the DC surface.4-6 Interestingly, DCs are ‘voracious eaters’ during their lifetime, feeding on soluble antigens by macropinocytosis and particulate antigens derived from particulate adjuvants and dying or apoptotic cells, and microorganisms from their immature to mature age by phagocytosis.7-10 Importantly, phagocytosis involves triggering of cell surface receptors that drive act in polymerization and active internalization of particles.11
While DC has been shown to be an efficient antigen sampling cell, its antigen targeting effect has been subject of interests especially due to its significance in the reduction of antigens required to stimulate an effective T cell response. Because antigen formulated in mineral adjuvants, micro particles, and nanoparticles is modified into particulate form and is well-targeted by DCs, these additives are the important therapeutic targets such as drugs and vaccines. These additives are able to act as an intracellular antigen depot providing a prolonged antigen presentation due to sustained release.12,13 Understanding how these additives will affect the function of DCs will be subject of interests for drug and vaccine designers. Thus, this study has been conducted to observe and analyze the role of BMDCs in taking up a soluble and particulate antigen and their efficiency in presentation in vitro.
In this study, Ealpha green fluorescence protein (EαGFP)/YAe system was used to visualize antigen uptake and presentation by BMDCs12,14 (Figure 1). This system allows assessment of antigen uptake/degradation and, in combination with the YAe antibody antigen presentation in situ.12,14,15When this antigen is internalized by DCs, EαGFP is degraded and the Eα peptide is presented by IAbMHCII on the cell surface. These Eα: IAbMHCII complexes can be detected by staining the cells with YAe antibody because this antibody can efficiently bind the complex of Eα(52-68) with IAbMHCII.14,16 Therefore, the YAe antibody sees what T cell receptor sees (Figure 1). In this assay, GFP signal inside cell suggests the antigen engulfed within cell and missing of this signal indicates its complete degradation. Thus, this direct, simple, and easy technique was used in the observation of phagocytosis and pinocytosis and presentation of soluble antigen by BMDCs in vitro.
Preparation of DCs from murine bone marrow: marrow cells (2×106/well) from six- to eight-week-old C57BL/6 (H-2b/b) mice were used to prepare BMDCs using 10% GMCSF (63x cell line supernatants) supplemented with RPMI [RPMI 1640 (Sigma, UK), 10% FCS (Gibco, UK), 100μg/mL penicillin and streptomycin (Invitrogen, UK) and 100 μg/mL l-glutamate (Invitrogen, UK) as described previously.12,17
Antigens: The fluorescent antigen, EαGFP, was prepared using methods described previously.12,15 EαGFP is a chimeric fluorescent protein antigen15 produced from a genetic fusion of Eα peptide to GFP.18 Eα peptide (amino acid residues 52–68) is the immune dominant epitope derived from IEd alpha chain and presented by IAb–restricted MHC class II molecules.14,16,19
Particulate beads uptake by DCs: To study phagocytosis of bead and antigen targeting to BMDCs, cells were incubated in media, in the Phycoerythrin (PE)-conjugated carboxylate-modified microspheres (2µm) (2µL beads) (Invitrogen), Bead-Mix [EαGFP and Phycoerythrin (PE)-conjugated beads; 2µL beads containing 2.5x106 beads per 0.5x106 DCs/well], or EαGFP (50µg/mL) alone for 1hour or 24hours and were analyzed by flow cytometry.
Cells were collected in 5mL FACS tubes (BD FALCON, BD Biosciences Discovery Lab Ware) and washed (400xg, 5minutes, 4°C) in FACS buffer (5% FCS, 0.1% sodium azide) and incubated with either purified anti-mouse CD16/CD32 (1 in 100 dilution) or 100µL Fc block (2.4G2 hybridoma supernatant) for 30minutes to prevent non-specific binding via Fc receptors. Then, cells were stained with fluorophore-labeled antibody (biotinalyted anti-mouse Eα52 - 68, eBiosciences, clone: eBioYAe, cat no. 13-5741, bio anti-mouse IgG2b, Southern Biotech, clone: A-1, cat. 0104-08, allophycocyanin (APC) streptavidin, eBiosciences, cat no. 17-4317), and incubated for 30minutes. Cells were washed with FACS buffer twice (400xg, 5minutes, 4°C) and analyzed on a FACS Caliber (BD Biosciences). The results of flow cytometry were analyzed by FlowJo software (FlowJo 8.7.1, Stanford University 1995-96).
Similarly, some cells were incubated with beads, Bead-Mix or EαGFP in chamber slides (LabTek) for 1hour and 24hours and processed for biotyramide system (BTS). Then, cells in chamber slides were washed with Fc block, Avidin block, Biotin block (Vector Laboratories, Inc. Burlingame, CA) and stained with YAe-Bio at 1:100 dilution (Biotin conjugated anti-mouse Eα52-68peptide, YAe-bio, 0.5mg/mL, eBiosciences) diluted in TNB Blocking Buffer (PerkinElmer Life Sciences). The cells were stained with Streptavidin-horse radish peroxidise (HRP) (1.2mL, PerkinElmer Life Sciences, Inc. Boston, MA, US) at the 1:100 dilution in TNB Blocking Buffer, then with biotyramide (diluted in 1:50 in Amplification Buffer, Molecular Probes, Invitrogen) and finally with Streptavidin AF 647 (Alexa Fluor 647 conjugated/tagged, Invitrogen). Finally, one drop of 4’, 6-diamidino-2-phenylindole (DAPI) (Vector laboratories, Inc. Burlingame, CA) was used in each well and 25x60mm coverslip (Borosilicate Glass, VWR International) was used. Cells were observed by confocal microscopy (LSM 510 software, Carl Zeiss). Images were analyzed by Velocity Software (Velocity 4.1, Improvision, Viscount Centre, University of Warwick Science Park, and Coventry).
Antigen uptake and presenting assay by flow cytometry: Flow cytometry was used to evaluate the morphologic and cellular characteristics on the basis of the Forward Scatter Characteristics (FSC) and Side Scatter Characteristics (SSC). Cells were studied by relative size based on FSC and relative granularity based on SSC. There was no difference in FSC or SSC in cells incubated in bead or bead-mix compared in cells incubated in media or EαGFP (Figure 2). However, cells that captured beads were easy to detect due to high signals of PE at various series in flow cytometry histograms. Pinocytic activity of cells was calculated by analyzing the % of cells that have GFP signals in Fluorescein Isothiocyanate (FITC) background. Similarly, phagocytic activity was calculated by analyzing the % of cells that have PE-conjugated beads (Figure 2).
In this study, the efficiency of BMDCs in pinocytosis and phagocytosis was compared at 1hour and 24hours following incubation with EαGFP or bead, or bead-mix (Figure 2). Interestingly, BMDCs were highly efficient in phagocytosis of beads and this efficiency was increased with respect to duration of incubation (Supplementary Figure 1A). The data also showed that both % of GFP-positive cells (Supplementary Figure 1B) and % of YAe-positive cells (9 fold increase) were increased from 1hour to 24hours indicating BMDCs efficiently pinocytose soluble antigens and present them in the context of Eα:IAbMHCII complexes with an increase in duration of incubation. However, total GFP-positive cells (Supplementary Figure 1B) and cells that contain both phagocytozed beads and GFP (Supplementary Figure 1C) were very low in the Bead-Mix treatment group at 1hour as well as 24hours. However, cells that contain both phagocytozed beads and YAe molecules sharply increased from 1hour to 24hours compared with YAe molecules in EαGFP-treated cells (Supplementary Figure 1D). These results suggest that BMDCs efficiently engulf MPs and soluble antigens and enhances processing of soluble antigens in the presence of MPs; however further a pulse chase assay should be conducted to validate this hypothesis.
Phagocytosis and antigen presenting assay by confocal microscopy: Next, BTS and confocal microscopy were used to visualize antigen capturing and antigen presenting efficiencies of BMDCs (Figure 3). These figures confirm that DAPI, PE-labeled beads, and EαGFP are highly effective materials in visualizing antigen engulfed by BMDCs. Clear indications of more than 20 beads in some DCs were observed indicating some DCs are highly phagocytic or voracious upon MPs (Figure 3A and 3B). With the help of appropriate staining, presentation of antigen by BMDCs could be observed (Figure 3C). Overall, the data indicate that biotyramide assay and EαGFP/YAe assays could be directly and reliably used to observe particulate capturing behavior and antigen presenting characteristics of BMDCs in vitro.
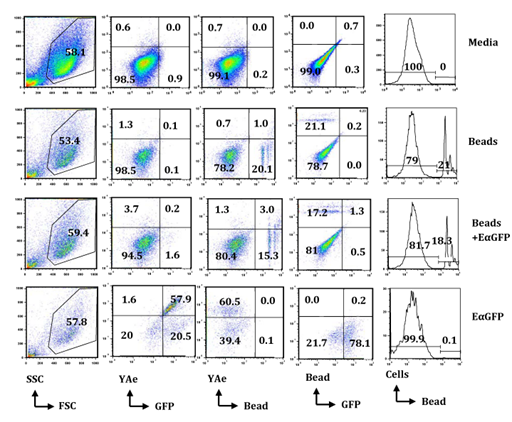
Figure 2 BMDCs are efficient in uptake of soluble antigen as well as particulate such as bead (MP) and in presentation of soluble antigen in vitro: BMDCs (0.5x106/5mL) obtained from C57BL/6 mice were incubated in media, EαGFP (50µg/mL), or EαGFP (50.0µg/mL) mixed in bead for 1hour and 24hours. Cells were stained with YAe-bio antibody and APC Streptavidin and processed for flow cytometry analysis. A total of 50,000 cells were collected based on FSC and SSC. Dot plot shows the proportion of cells positive for YAe vs. GFP, YAe vs. bead and GFP vs. bead. Histogram plots show the % maximum of cells positive for PE-conjugated beads (MPs). All these figures are the representative diagrams obtained by analysis of cells incubated for 24hours.
The use of flow cytometry and confocal microscopy in the characterization of various particles and their roles in antigen internalization by APCs have been already discussed.20-26 In contrast to these studies, the current study employs YAe/EαGFP system to detect unprocessed or native antigens in the form of GFP and processed and presented antigens in the form of Eα:IAbMHCII complexes that are recognized by YAe antibody. For this system, flow cytometry is crucial. Flow cytometry is an exceptional method to detect, quantify, and characterize MPs in physiologic and in pathologic conditions.26 The current study shows that MPs reduced an uptake of soluble antigens and enhanced the presentation of soluble antigen with respect to time. This result may be attributed to an enhanced antigen processing efficiency of the current 2µm MPs, however it is difficult to generalize in the absence of a pulse chase experiment. Previous reports have shown that compared to 1µm particle, only 20nm particles reduced antigen degradation via interference in the lysosomal compartment.23 In an in vivo experiment, negatively charged 20nm particles were efficient in enhancing antigen presentation to CD4+ T cells in lung-draining lymph nodes.21 However, glycine-coated 50nm particles down regulated the stimulatory property of CD11b+ DCs in lung draining lymph nodes to OVA-specific T cells.27 Brewer and colleagues have shown that compared to antigen prepared in small vesicles (<200nm), antigen prepared in large vesicles (200nm - 560nm) tends to target early phagosomes and generated an enhanced T cell activation via MHCII pathway.20 This suggests the crucial role of particle sizes on antigen degradation and consequently on DC function. All these different results might be due to dissimilar particles, their sizes, functional groups and charges on these particles, APC subsets, and environment of the study performed (in vitro vs in vivo). Thus, a pulse chase experiment and flow cytometry analysis will be a beautiful model of the prediction of antigen uptake, enzymatic degradation, and antigen presentation. In this context, the EαGFP/YAe system and MP uptake protocol will assist to elaborate the lifecycle of particulate antigen inside DCs.12
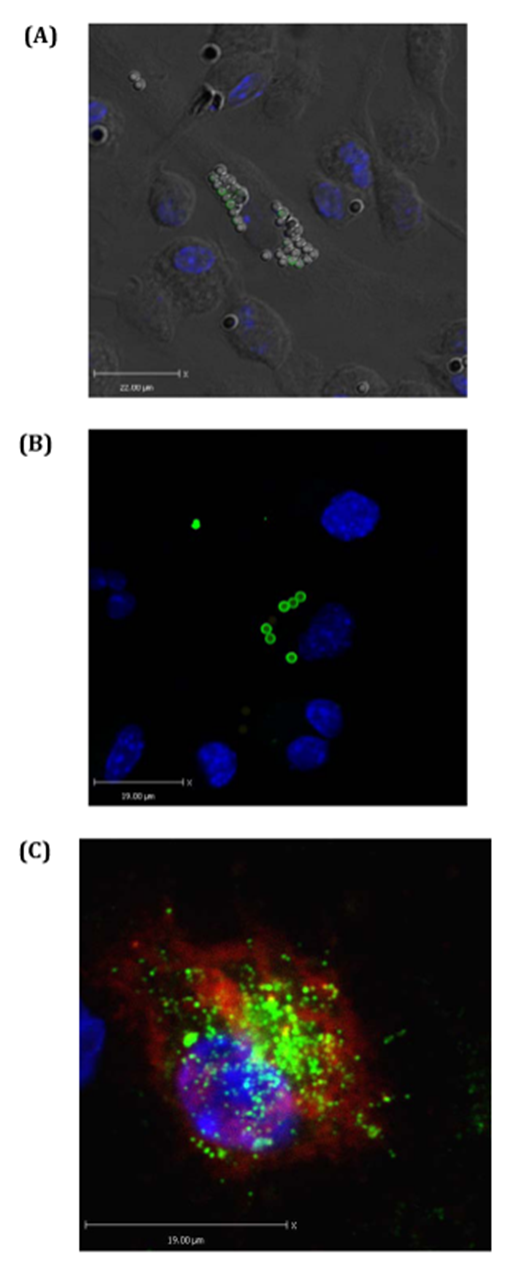
Figure 3 Particulates (MPs) target antigen to BMDCs in vitro: BMDCs were treated with EαGFP and or 2micron beads and incubated for 1hour in chamber slides. Biotyramide assay was used for confocal microscopy. (A) Shows BMDCs one of which has voraciously engulfed beads (MPs). Some of these beads still have GFP signals. Nucleus is stained by DAPI that excites at 405nm and gives blue color. (B) Shows some BMDCs one of which is voracious feeder on bead-mix. This cell also contains EαGFP-nonconjugated bead inside it. (C) Represents the BMDC that has pinocytosed soluble EαGFP antigen and presented it in the context of Eα:IAbMHCII complexes as detected by YAe antibody. YAe signals (red signal) are amplified due to binding of Streptavidin 647 which excites at far red.
Interestingly, confocal data suggest the voraciousness of BMDCs in vitro. These cells efficiently captured particulates and this efficiency increased with time suggesting the consistence voraciousness of these cells. Confocal microscopy uses BTS that includes of the use of HRP to catalyze the remains of the biotin-labeled tyramide (amplification reagent). It is crucial to note that BTS efficiently enhances both chromogenic and fluorescent signals and therefore has been a beautiful tool in the visualization of uptake of both particulate and soluble antigen and their presentation on cell surface.
In conclusion, drug delivery using particulates coupled with EαGFP/YAe protocol for flow cytometry and confocal microscopy will be effective to study the antigen sampling and antigen presenting characteristics of BMDCs in the presence or absence of particles in vitro. This cellular pathway of BMDCs can be targeted to devise a novel approach for the rational design of drugs and vaccines in future.
The present study showed the pharmacological potential of the ethanolic extract of Neem bark. Our findings demonstrated that the F-EtOAc, obtained after saponification of EtCNeem, showed to be rich in phenolic and flavonoid compounds with antioxidant potential, as well as a nontoxic.
The author declares no financial or commercial conflict of interest. This work was conducted in the University of Strathclyde, Scotland. For this work, the author has no relation with the current affiliation as shown in the first page.

©2014 Ghimire. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.