Journal of
eISSN: 2377-4282


Nanotechnology is one of the growing fields in medicine. “Nano” stands for the particle size ranging from 1–1000µm. Nanosuspensions are the sophisticated technology in the field of nano science. A pharmaceutical nanosuspension is defined as a very finely colloid, biphasic, dispersed, solid drug particles in aqueous vehicle, size below 1μm, without any matrix material, stabilized by surfactants & polymers, prepared by suitable methods for drug delivery applications, through various routes of administration like oral, topical, parenteral, ocular & pulmonary routes. A nanosuspension not only solves the problems of poor solubility and bioavailability, but also alters the pharmacokinetics of drug and thus improves drug safety and efficacy.
Keywords: nanosuspension, drug nanocrystals, application
TPGS: Tocopheryl Polyethylene Glycol Succinate; HPMC: Hydroxyl Propyl Methyl Cellulose; SC: Stratum Corneum; PEG: Polyethylene Glycol
During the last two decades, many modern technologies have been established in the pharmaceutical research and development area. The automation of the drug discovery process by technologies such as high–throughput screening, combinatorial chemistry, and computer–aided drug design is leading to a vast number of drug candidates possessing a very good efficacy.1 Unfortunately, many of these drug candidates are exhibiting poor aqueous solubility. According to estimates, about 40% of the drugs in the pipelines have solubility problems.2 The increased use of high throughput screening methods leads to the discovery of more drugs being poorly water soluble. In the literature, figures are quoted that about 60 percent of the drugs coming directly from synthesis are nowadays poorly soluble.2 Poor solubility is not only a problem for the formulation development and clinical testing; it is also an obstacle at the very beginning when screening new compounds for pharmacological activity. Long before one of these compounds can reach the market; it needs to be formulated for the pharmacological activity tests and for the preclinical studies. From this, there is a definite need for smart technological formulation approaches to make such poorly soluble drugs bioavailable. The great challenge for the pharmaceutical development is to create new formulation approaches and drug–delivery systems to overcome solubility problems of these drug candidates which are also often associated with poor oral bioavailability.1,3,4 Making such drugs bioavailable means that they show sufficiently high absorption after oral administration, or they can alternatively be injected intravenously.
There is quite a number of formulation approaches for poorly soluble drugs which can be specified as "specific approaches". These approaches are suitable for molecules having special properties with regard to their chemistry (e.g. solubility in certain organic media) or to the molecular size or conformation (e.g. molecules to be incorporated into the cyclodextrin ring structure). Of course it would be much smarter to have a "universal formulation approach" applicable to any molecule. Such a universal formulation approach to increase the oral bioavailability is micronization, meaning the transfer of drug powders into the size range between typically 1–10 µm.5,6 However, nowadays many drugs are so poorly soluble that micronization is not sufficient. The increase in surface area, and thus consequently in dissolution velocity, is not sufficient to overcome the bioavailability problems of very poorly soluble drugs of the biopharmaceutical specification class II. A consequent next step was to move from micronization to nanonization. Since the beginning of the 90s, the company Nanosystems propagated the use of nanocrystals (instead of micro crystals) for oral bioavailability enhancement, and also to use nanocrystals suspended in water (nanosuspensions) for intravenous or pulmonary drug delivery.2
Over the past two decades, drug nanocrystal technology has been undoubtedly the highlight in pharmaceutical field. One of its major contributions is the benefits that can be gained by formulating poorly soluble drug7,8 This approach generally produces dispersions of drug nanocrystals in a liquid medium (typically water), which are called “nanosuspensions”. Nanosuspensions consist essentially of pure drug nanoparticles (100–1000 nm) and a minimum amount of surface active agents required for stabilization. The solution was simple; in general, simple solutions possess the smartness that they can be realized easier than complex systems and introduction to the market is faster. Nevertheless, it took about ten years before the first nanocrystals in a tablet appeared on the market, the product Rapamune®.
At present, approaches developed to produce drug nanosuspensions mainly include the so called ‘bottom up’ (precipitation) and ‘top down’ (media milling, high pressure homogenization, etc.). The bottom up technology dissolves the drug in solvent, and then precipitates it by adding the solvent to a non–solvent. These techniques are not widely used because of some prerequisites, such as usage of organic solvents and the drug should be soluble at least in one solvent.9 The top down technologies are disintegration methods, and so can be employed for all insoluble drugs including ‘brick dust drugs’. Drug nanocrystals exhibit many advantages including high efficiency of drug loading, easy scale–up for manufacture, relatively low cost for preparation and applicability to various administration routes, such as oral.10 parenteral.11 ocular.12 and pulmonary delivery.13 All these advantages have so tremendous impacts on promoting drug nanocrystals successfully from experimental researches to patients that several products have been launched into market (Table 1).
|
Drug Compound |
Nano-Sizing Approach/ Technology |
Administration Route |
|
Griseofulvin |
Bottom up, coprecipitation |
Oral |
|
Nabilone |
Bottom up, coprecipitation |
Oral |
|
Sirolimus |
Top–down, media milling |
Oral |
|
Aprepitant |
Top–down, media milling |
Oral |
|
Fenofibrate |
Top–down, media milling |
Oral |
|
Megestrol acetate |
Top–down, media milling |
Oral |
|
Fenofibrate |
Top–down, media milling |
Oral |
|
Paliperidonepalmitate |
Top–down, media milling |
Injection |
|
Itraconazole |
High pressure homogenization |
Injection |
|
Paclitaxel |
High pressure homogenization |
Injection |
|
Paclitaxel |
Media milling |
Injection |
|
Paclitaxel |
Precipitation |
Injection |
|
Nimodipine |
High pressure homogenization |
Injection |
|
Oridonin |
High pressure homogenization |
Injection |
|
Curcumin |
High pressure homogenization |
Injection |
|
Melarsoprol |
High pressure homogenization |
Injection |
|
Naproxen |
Media milling |
Oral |
|
SU5416 |
Media milling |
Oral |
|
UG558 |
Media milling |
Oral |
|
Cilostazol |
Media milling |
Oral |
|
Itraconazole |
Spray freezing into liquid |
Pulmonary |
|
Cyclosporine |
High pressure homogenization |
Oral |
|
Spironolactone |
High pressure homogenization |
Oral |
Table 1 Key nanocrystal formulations in different administration routes based on drug nanoparticle technology.
During the last decade of the 20th century lots of experiments have been done to study the manufacturing technologies of drug nanocrystals and their in vitro physical and chemical properties, such as procedure parameters, formulation composition, physical and chemical stability, crystalline structure, enhanced saturation solubility and dissolution rate, bio adhesion and so on (Figure 1). Some published reviews have well summarized and discussed results of these researches from different aspects.4,9,14,15
The major advantages of nanosuspension technology are its general applicability to most drugs and its simplicity. Interesting special features of nanosuspensions are.16
Drug nanocrystals are crystals with a size in the nanometer range, meaning that they are nanoparticles with a crystalline character. There are discussions about the definition of a nanoparticle, referring to the size of a particle to be classified as a nanoparticle. Depending on the discipline, e.g. in colloid chemistry, particles are only considered as nanoparticles when they are in sizes below 100 nm or even below 20 nm. Based on the size unit, in the pharmaceutical area, nanoparticles should be defined as having a size between a few nanometers and 1000 nm; thus, micro particles possess consequently a size 1–1000 micrometer. A further characteristic is that drug nanocrystals are composed of 100% drug; there is no carrier material as in polymeric nanoparticles. Dispersion of drug nanocrystals in liquid media leads to "nanosuspensions", in contrast to "microsuspensions" or "macrosuspensions". In general, the dispersed particles need to be stabilized, e.g. by surfactants or polymeric stabilizers. Dispersion media can be water, aqueous solutions or non–aqueous media.e.g. liquid polyethylene glycol (PEG), oils.
Depending on the production technology, processing of drug micro crystals to drug nanoparticles can lead to either a crystalline or to an amorphous product, especially when applying precipitation. In the strict sense, such an amorphous drug nanoparticle should not be called nanocrystal. However, one often refers to "nanocrystals in the amorphous state".2
Drug nanocrystals are generally considered as a safe and well tolerated dosage form for many administration routes. Nano crystal formulations permit a more efficient oral administration due to two main mechanisms: improved solubility and dissolution rate and increased bioadhesion to the intestinal wall. Consequently when a drug is orally administered as nanocrystals the high drug concentration gradient between the gastrointestinal tract and blood vessel enhances absorption resulting in increased bioavailability. Currently many researches are focused on the development of new formulations for improving oral bioavailability using the nanosuspension technologies, besides the marketed products.17 Carrier–free nanocrystals, containing only 100% pure drug, can show advantages for ophthalmic administration, because they can minimize the irritation to eyes, consequently decreasing tearing and drainage of instilled dose. Currently there are a few studies investigating anti–inflammatory drugs in the form of nanocrystals for ocular application. Drug nanocrystals can be also incorporated into the water phase of topical formulations, both cosmetic forms, such as sun screen, anti–aging products, and pharmaceutical dermal preparations, as anti–inflammatory creams or gels. Dermal nanocrystals are on the market for cosmetic applications. For example, rut in submicron crystals are in the JUVEDICAL line of Juvena Switzerland.18,19
Recently targeted formulations using surface modified nanosuspensions have been developed. Improvements of pharmacological effects due to drug nanocrystals, might be obtained from their ability to deliver drugs directly to the diseased tissues, such as infected macrophages, tumours and brain. Delivery to target cells and cellular internalization of nano particles is under investigation using nanosuspensions.2,16,20 Therefore nanocrystals are a strategy able to solve problems deriving from the current dosage forms. Until now a lot of formulations have been developed with nanosuspension technologies by various research groups, but only a few products have reached the marketplace, especially for the oral administration. The reason may be related to commercial and not only technical aspects, because it is difficult to replace a well–selling product with a nanosuspension, considering the high cost of market introduction. Moreover with a new molecule, an established routine delivery technology might be preferred. In conclusion, nanosuspension technology represents a versatile platform to produce safe and high quality formulations for poorly soluble active molecules.20
Oral and parenteral/intravenous routes are the ones in which developments are focusing, clearly due to the commercial background and the relation between the development costs for a market product versus its potential annual sales. However, drug delivery could also be improved when using drug nanocrystals for pulmonary and ophthalmic administration or dermal application.
Nanosuspension for pulmonary drug delivery
Pulmonary drug delivery to the lung represents a non– invasive possibility for local and systemic therapies. Direct pulmonary delivery in humans can be achieved using an aerosol generated by either an inhaler or nebulizer. The local application of therapeutic agents to the lungs has the advantages of an increased selectivity and high local concentration over other routes of administration, thus being the prevalent approach for the treatment of respiratory diseases. On the other hand, pulmonary route has gained growing attention as a potential way for systemic drug delivery, due to the large alveolar surface area, the thinness of the epithelial barrier and extensive Vascularization suitable for drug absorption.2,21,22
Moreover, drugs administered through the pulmonary route to reach the systemic circulation, avoid the first–pass metabolism of the gastrointestinal tract, and may eliminate the barrier to patient compliance caused by injections. However, the fate of the medication administered by this route is highly variable depending on parameters such as the particle size of the aerosol, particle morphology and geometry, surface adhesive properties and mechanism and rate of elimination from the respiratory tract.23 Among the properties of the drug formulation, the aerodynamic diameter of the aerosol describes its aerodynamic behavior with consideration of size, density and shape, and its optimization is of primary importance for a successful pulmonary delivery.24
In fact, different mechanisms govern the pulmonary deposition of inhaled particles depending on the aerodynamic diameter, and values between 1 to 5 µm are required to target the lower respiratory region. When drug nanocrystals in aerosol droplets deposit in the lung, they can spread more evenly on the lung surface, especially when coated stabilizers have good spread ability. Then drug nanocrystals dissolve rapidly in the lung lining fluid leading to a high concentration due to their nano–range size. This is very helpful for localized treatment or prophylaxis of respiratory diseases. In humans, orally inhaled aerosols greater than 5–10 µm are mostly deposited in the oropharynx and incapable of reaching the lung, while the finest aerosols (<1µm) are mostly exhaled without deposition.25 Many studies have demonstrated that pulmonary delivery of nanosuspensions favor higher lung tissue concentrations and markedly raise the lung to serum ratio of drugs compared with other administration route.26–29 Beside this, this is also conducive to a rapid onset of systemic effect.22,23,30 Because high drug concentration in the alveoli would rise the driving force for permeation through the alveolar membrane, and result in higher Cmax and earlier Tmax.6,18,31
When nanosuspensions contain particles in amorphous form characterized by higher inner energy, this effect is more obvious.32 However, in spite of these advantages, there are also some notable issues in pulmonary delivery of drug nanosuspensions. Nebulization is a complex process affected by the characteristics of the nebulizer and compressor, droplet size, properties of the formulation, breathing pattern of the patient and respiratory tree anatomy.31 In addition, types of surface coatings of nanocrystals may also influence the safety of pulmonary application.
Unfortunately, this correlation between aerosol size and its deposition within the lung is not applicable to small laboratory rodents, such as rats and mice, primarily due to their nose–breathing nature. Thus, more sophisticated in vivo models (Table 2) than natural nose–breathing inhalation have been developed to assess pulmonary distribution and biopharmaceutical properties of novel drug formulations (eg. Intratracheal instillation, intubation followed by aerosol exposure).18,33
|
Administration Route |
Drug |
Method of manufacturing |
Results |
Model |
|
Dermal |
Rutin, Hesperidin |
Wet milling and high pressure homogenization |
Increased antioxidant activity compared to solution of water soluble derivative |
In vivo (human) |
|
Lutein |
High pressure homogenization |
Higher drug accumulation into the skin compared to coarse suspension |
In vitro (porcine ear skin) |
|
|
Diclofenac sodium |
Controlled crystallization during freeze drying |
Increased drug flux through the skin compared to coarse suspensions |
In vitro (Micropig skin) |
|
|
Ibuprofen |
Wet milling |
Increased drug flux through skin as function of nano crystal size and formulation components |
In vitro (human &porcine skin) |
|
|
Tretinoin |
Liquid solvent antisolvent precipitation |
Improved drug stability and dermal delivery in comparison with solution and nanoemulsion |
In vitro (porcine skin) |
|
|
Ocular |
Hydrocortisone, Prednisolone, Dexamethasone |
High pressure homogenization |
Increased drug action, higher absorption compared to solution and microcrystalline suspension |
In vivo (rabbits) |
|
Hydrocortisone |
Wet milling, Micro-fluidic precipitation |
Prolonged drug action compared to solution |
In vivo (rabbits) |
|
|
Brinzolamide |
Wet milling |
Drug action comparable to drug solution |
In vivo (rats) |
|
|
Forskolin |
Wet milling |
Prolonged drug action and corneal retention |
In vivo (rabbits) |
|
|
Cyclosporin A |
In situ formation |
Higher corneal concentration compared to solution and micro emulsion |
In vitro (porcine eyes) |
|
|
Pulmonary |
Fluticasone, Budesonide |
Wet milling |
Prolonged drug retention in the lungs as compared to solutions |
In vivo (rats, Intratracheal) |
|
Fluticasone |
Wet milling |
Efficient lung deposition after aerosol delivery |
In vivo (mice, nasal ) |
|
|
Baicalein |
Combination (antisolvent recrystallization + high pressure homogenizer |
Comparable plasma concentration when administered as aerosol or intravenous |
In vivo (rats, Intratracheal) |
|
|
Itraconazole |
Wet milling |
Prolonged deposition time in the lungs |
In vivo |
|
|
Budesonide |
Not specified |
Plasma concentration comparable to suspension, faster Tmax and higher Cmax |
In vivo (human) |
|
|
Budesonide |
Not specified |
Faster absorption and higher Cmax compared to commercial suspension |
In vivo (human) |
Table 2 Literature examples of drug nanocrystal formulations for topical/mucosal application 2.
Formulations containing nanoparticulates have demonstrated many advantages in pulmonary route, among them drug nanocrystals show a great potential for pulmonary delivery of poorly soluble drugs.23,34. Two kinds of pulmonary formulations containing drug nanocrystals have been reported. One is aqueous nanosuspensions packaged and administered by a nebulizer. Drug nanocrystals can be collected and transported into the lung by the small aerosol droplets generated by the nebulizer. The other one is inhaled powders containing dried nanocrystals dispersed in some inhalable carriers (e.g. inhalable lactose).35 These powders can be inhaled by commercial dry powder inhalers. Compared to the conventional microparticles for pulmonary use, nanoparticles offer a more homogenous distribution of drugs in the nebulized droplet or among the inhalable carriers.
In the last decades, different kinds of micro– and nano–sized vehicles have attracted growing attention as potential drug carriers for pulmonary delivery (Table 2). Poorly soluble drugs could be inhaled as drug nanosuspension. The drug nanosuspension can be nebulized using commercially available nebulizers.36 Nanocarriers in general offer the advantage of a precise control of particle size to reduce the variability in lung absorption, with further possibilities of prolonged residence time, controlled release and targeting properties.37
Nanocrystal suspensions in particular provide several advantages compared to typical formulations as solutions and dry powders. In the past, most of these drugs were administered as aerosols, but the use of chlorofluorocarbon (CFC) must be avoided. Therefore, alternatives such as dry powder inhalers or metered dose inhalers without CFC (MDI) were developed. These systems are filled with micronized drug powders produced by jet–milling. The mean particle size in the lower micrometer range (3–25 μm) results in a significant oropharyngeal deposition of larger particles leading to increased occurrence of candidasis. Additionally, the oral deposition of the drug leads to gastrointestinal (GI) drug absorption followed by systemic side effects.
Nanosuspensions can be successfully applied to overcome these problems. The nebulization of nanosuspensions generates aerosol droplets of the preferred size loaded with a large amount of drug nanocrystals. Ostrander expressed that using nebulized nanosuspensions, the respirable fraction is distinctly increased in comparison to conventional MDIs. The smaller the particle size of the drug nanocrystals, the higher the drug loading of the aerosol droplets.23,38 Therefore, the required nebulization time is distinctly reduced.31 Beside this, drug nanocrystals show increased muco adhesiveness, leading to a prolonged residence time at the mucosal surface of the lung. Nanosuspensions are suitable to formulate poorly soluble drugs without the extensive use of organic co–solvents, thus reducing their in vivo interference and potential toxicological effects. Moreover, the concentration of the active principle in the nanosuspension is not limited by solubility in the vehicle, thus permitting to reach a wider dose range.
Compared to dry powder formulations, nanosuspensions consist of prewetted particles that may improve dissolution and consequent lung absorption, though maintaining the solubility–dependent prolonged release from nanocrystals.18,23 Disposition in the lungs can be controlled via the size distribution of the generated aerosol droplets. Compared with micro crystals, the drug is more evenly distributed in the droplets when using a nanosuspension. The numbers of crystals are higher; consequently, the possibility that one or more drug crystals are present in each droplet is higher.39 Delivery of water–insoluble drugs to the respiratory tract is very important for the local or systemic treatment of diseases. Many important drugs for pulmonary delivery show poor solubility simultaneously in water and nonaqueous media, for example, important corticosteroids such as budesonide or become thasonedipropionate.
As reported, nanocrystal suspensions have the advantage of excluding any matrix and polymeric material usually included in other nanocarriers. This is of highest importance in pulmonary drug delivery since the deposition of nanoparticles on lungs surface have shown a variable toxicological potential, dependant on matrix degradability and chemical composition.22 Several authors described the properties of nanosuspensions for lung delivery when compared to solutions or coarse suspensions of the same drug.
Nanosuspensions of fluticasone and budesonide prepared using a wet milling technique, showed prolonged drug retention in the lungs as compared to solutions in vivo, with a difference between the two drugs which can be ascribed to their different solubility.23 Another study ruled out the possibility of administering fluticasone coarse suspensions because of unacceptable aerodynamic properties of particles. Conversely, efficient delivery and dose–dependent lung deposition were achieved after administration of nanosuspension aerosol to mice in vivo.40
Zhang et al. compared the bioavailability of baicalein in rats after pulmonary administration vs intravenous injection. A baicalein nanosuspension was formulated using a combination of antisolvent recrystallization and high pressure homogenization methods. It was administered to the lungs and compared to an intravenous injection of a baicalein solution. The different formulations show edno significant difference in pharmacokinetic parameters, thus providing an example of potential systemic administration through pulmonary route.33 Tolerability of nanosuspensions after repeated dose should also be assessed, especially for those therapeutic agents that are required for long term therapies.
Recently, Rundfeldt et al39 described a nanosuspension of an it raconazole as potential treatment for broncho pulmonary aspergillosis. The nanosuspension was produced using a wet milling technique, and the final formulation was screened for the content of inorganic contamination potentially derived from the grinding media, since inorganic contaminations are not acceptable for inhaled preparations. The inhalation of the nanosuspension resulted in a long lasting pulmonary exposure to the drug, with an Itraconazole lung concentration still higher (28–fold) than the MIC of Aspergillus fumigatus at 24 h from the inhalation. Moreover, repeated exposures to the nanosuspension (once daily for 7 days) showed no histological or behavioral changes in rats.40 Due to the promising results in preclinical animal studies, the pharmacokinetics of nanosuspensions after pulmonary administration was explored on human volunteers.
In a trial on healthy adult volunteers reported by Kraft et al.31 budesonide nanosuspension was compared to a commercial regular suspension of the same drug, reporting comparable plasma AUC but higher Cmax and shorter Tmax. These results are in accordance with the more rapid rate of solubility and subsequent absorption of nanocrystals compared to bigger particles.31 In 2009, Shrewsbury et al.41 conducted a similar study on a novel submicron suspension of budesonide in clinical development at that time, obtaining comparable results on adult volunteers. The cited formulation reached an initial phase III clinical trial on children for potential treatment of paediatric asthma, but failed in achieving a significant difference compared to placebo in symptoms improvement.
Nanosuspension for ocular drug delivery
The topical ocular administration of drugs is the most common route to provide treatment for both superficial and intraocular diseases. Depending on the target sites of the different ocular pathologies, drugs either need to be retained at the cornea and/or conjunctiva(e.g., conjunctivitis, blepharitis, keratitis sicca) or cross these barriers and reach the internal structures of the eye (e.g., glaucoma, uveitis) (Figure 2). However, ocular bioavailability of the instilled drugs is known to be low (<5%) due to the many biological processes and physical barriers present in the eye.42,43 The drug, being diluted with the lachrymal fluid immediately after application, remains in the precorneal space for a very short period of time because of the continuous fluid drainage, the blinking reflex and the activity of metabolic enzymes, which may significantly degrade the drug molecules. During this time, drugs targeting the inner eye have to cross one of the barriers covering the eyeball: the cornea and/or the conjunctiva. The cornea is the transparent, dome–shaped front part of the eye that covers the iris and pupil. This structure is composed of an outer stratified epithelium which covers a thicker layer of collagen fibers in a glycoproteic matrix, and it is separated from the aqueous humor by the corneal endothelium.44 The cornea lacks blood and lymphatic vessels, so it would be the optimal way to deliver drug into the eye without systemic absorption. Unfortunately, the transport across this barrier is severely limited by the presence of tight junctions that hinder the paracellular pathway, followed by most of the small hydrophilic molecules administered as eye–drops. The conjunctiva is composed by a stratified epithelium which covers the eyeball (beginning at the outer edge of the cornea) and lines at the inside of the eyelids. This structure is more permeable than the cornea for drugs topically applied into the eye, but the entry through this way is normally associated with systemic drug absorption which leads to poor distribution in the ocular tissues and increases the risk for systemic side effects.45 Moreover, drugs intended to treat pathologies related to the back of the eye (eg. macular degeneration) have to diffuse through the vitreous humor, a gelled matrix of collagen fibrils and glycosaminoglycans, to reach the retina (Figure 2).
Most of ophthalmic diseases are treated with topical application of eye drops (solution or microsuspensions). In conventional eye drops, ocular bioavailability is low due to the rapid and extensive drug loss caused by drainage through the nasolacrimal duct and blinking. Frequent instillations are necessary in order to maintain therapeutic effect, which in turn leads to poor patient compliance or undesirable side effects from unwanted systemic drug absorption.
For ocular delivery of poorly soluble drugs, because the organic solvents and extreme pH should be avoid when considering the high sensitivity of the eye tissue, preparations such as microsuspensions and ointments have been developed to meet the therapeutic requires.46 These preparations are often accompanied by several issues such as irritation, blurred vision, and deficient concentration due to the limited drug solubility in lachrymal fluids and various anatomical barriers.47 Many studies have shown that several nanotechnological approaches (liposomes, polymeric nanoparticles, nanocapsules, polymeric micelles etc.) have been investigated to improve drug retention and corneal permeation, thus increasing ocular drug bioavailability.22,48
Among them, carrier–free nanocrystals exhibit more advantages. Containing only pure drug particles in nano–size range, nanosuspensions minimize the irritation to eyes, consequently decrease tearing and drainage of instilled dose.49 Higher solubility and dissolution rate due to the huge surface area can promote or facilitate transfer of drug molecules from the tear phase into the eye tissue50 Nanocrystals may be used not only to increase solubility in lachrymal fluids of poorly soluble drugs, but also to produce adhesive properties (determined by the nature of the surfactant in the formulation) that can be exploited for improving the retention and penetration of drugs into the eye. Nonionic surfactants (polysorbates and poloxamers) are of most common use in topical eye formulations since they are generally less irritating than ionic surfactants.12,51 In addition, to enhance the viscosity of the nanoparticles and strengthen the bio adhesion to the eye surface, nanoparticles can be incorporated into a suitable mucoadhesive base or ocular inserts to further prolong retention time and achieve a sustained release of the drug for a stipulated time period. Nanosuspensions of different viscosity were prepared by Kassem by incorporating the nanoparticles into different concentrations of hydroxyethyl cellulose. The results found that the AUC and MRT were markedly enhanced with the increase of the viscosity. Studies by Pignatello.12 also found that after surface modification by bio adhesive hydrophilic polymer, flurbiprofen and ibuprofen nanosuspensions demonstrated a good effect of sustained–release following ocular administration.
Dermal nanosuspensions are mainly of interest if conventional formulation technology fails or if it is distinctly less efficient. Dermal drug nanosuspensions lead to a supersaturated system because of their increased saturation solubility. The higher concentration gradient between topical formulation and skin can improve drug penetration into the skin. In addition, because of their small size, drug nanocrystals could target the hair follicle by protruding into the gap around the hairs. This was illustrated in solid lipid nanoparticles of a similar size. Adhesive properties of drug nanocrystals are also an area of interest. Adherence to the skin reduces the "loss" of drug to the environment/third persons. This is especially so in the event that highly active compounds are applied, e.g. hormones. For this reason, the drug estradiole was incorporated into solid lipid nanoparticles to better localize it on the skin.52–56
In the following section, recent examples of nanocrystal suspensions as ophthalmic drug delivery systems are provided (Table 2). Kassem reported an increased activity of corticosteroid nanosuspensions compared to micro–crystalline suspensions and solution on a rabbit animal model in vivo. In the same study, the viscosity of nanosuspensions, prepared by the high–pressure homogenization method, was evaluated and found to be of significant importance, especially in increasing the duration of drug action.
More recently, two hydrocortisone nanocrystal suspensions obtained by different methods of preparation (wet milling and microfluidic precipitation), achieved comparable AUC and showed a sustained drug action up to 9 h compared to 5 h of the drug solution. Not surprisingly, the milled hydrocortisone nanosuspension showed a superior stability after storage at room temperature for two months, as compared to the precipitated formulation. A nanosuspension of brinzolamide was prepared using a top down wet milling technique, and showed comparable results to a commercially available solution regarding in vitro cytotoxicity and in vivo activity on a rat ocular hypertension model.The concept of nanosuspensions for ocular drug delivery was also combined with other technological strategies to further increase drug residence time on the eye surface. For skolin nanocrystals were included in a liquid vehicle which readily forms a gel at the eye pH/temperature, and is capable of reducing intraocular pressure up to 12 h due to the combined properties of nanocrystal structure and mucoadhesive polymer. Luschmann developed a formulation which can precipitate cyclosporine–A nanocrystals when topically administered to the eye. The formulation showed no toxicity on corneal epithelial cells, and the in situ formation of a nanosuspension significantly increases the drug absorption as compared to a commercial micro emulsion. The major advantage of this kind of formulation is the formation of nanocrystals directly into the site of administration, thus having the superior skills of a nanosuspension with the easier preparative step of a solution.
Nanosuspension for topical/dermal drug delivery
Foldvari explained the skin represents an important site for non–invasive and painless delivery of therapeutic agents with possibility to control their release and to avoid the first–pass metabolism. After dermal absorption, drugs can reach different target sites and exert topical, regional, or systemic effects. For these reasons, delivery of drugs through the skin has been considered an attractive as well as a challenging research area. The primary challenge is to overcome the unusual impermeability of the skin barrier. The skin is a complex structure that protects the human body from invasion of pathogens, fends off chemical and physical assaults and controls essential processes like thermal regulation. It is made up of several anatomically distinct layers, namely the stratum corneum (SC), the avilable epidermis and the dermis. The physical barrier is mainly localized in the SC and consists of protein–enriched dead cells (corneocytes with cornified envelope and cytoskeletal elements, as well as corneodesmosomes) and lipid–enriched intercellular domains which are organized as multiple lipid bilayers (lamellar sheets composed of approximately equimolar concentrations of free fatty acids, cholesterol, and long chain ceramides). The nucleated epidermis also contributes to the barrier through tight, gap and adherent junctions, as well as through desmosomes and cytoskeletal elements. Therefore, the skin barrier function is not only dependent on one single component but on its total architecture. Appendages such as sweat glands, pilosebaceous units, and hair follicles are structures originating either from the dermis or the subcutaneous fat tissue which penetrate the skin thus forming important discontinuities in this compact structure.
Drugs applied to the skin surface enter the skin by a passive diffusion process through two pathways such as the transappendegeal and the transepidermal routes (Figure 3). The skin appendages present an alternative pathway for drug delivery into the skin, raising the possibility of avoiding the difficulty of delivering drug through the SC. This route was traditionally considered of minor importance because of the relatively small area, but recent results on follicular penetration have emphasized that the hair follicle pathways may play a major role in skin penetration pathway and reservoir of topically applied molecules. Moreover, the hair follicles and sebaceous glands are associated with various dermatological disorders such asacne, alopecia, and several skin tumors. Through the epidermis, there are two different routes by which a molecule can cross this barrier: the Trans cellular, across the corneocytes and the intercellular across the lipid domains between the corneocytes. Although it is generally accepted that the intercellular route provides the principal pathway for the permeation of drugs, this route is severely limited by the inability of the large majority of drugs to cross the skin at therapeutic rates due to the great barrier imposed by the outer SC layer. To increase skin permeability, a number of different approaches have been studied, ranging from chemical enhancers to soft matter nanocarriers such as liposomes, which when opportunely formulated might have the potential to meet the demands.
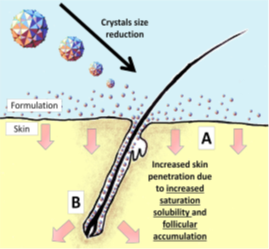
Figure 3 Proposed mechanism of increased dermal penetration of drug actives formulated as nanocrystals via (A) increased penetration into skin surface (epidermis) and (B) localization of nanocrystals in the hair follicles.
Dermal nanosuspensions are mainly of interest if conventional formulation approaches fail. The use of drug nanocrystals leads to an increased concentration gradient between the formulation and the skin. The increased saturation solubility leads to “supersaturated” formulations, enhancing the drug absorption through the skin. This effect can further be enhanced by the use of positively charged polymers as stabilizers for the drug nanocrystals. The opposite charge leads to an increased affinity of the drug nanocrystals to the negatively charged stratum corneum. Among the various strategies for enhancing dermal application, nanocrystals can be considered a rather new but highly interesting approach. Despite several nanosuspension products have become easily available in the last 10years, with most of them intended for oral administration, only few researches have been dedicated to the study of skin permeation and accumulation of drugs formulated as nanosuspension (Table 2).
However, the few published results clearly indicate that this technology could be very effective for improving dermal bioavailability of actives with both poor and more recently, medium solubility. Indeed, besides the increased saturation solubility and dissolution rate, nanocrystals also exhibit the property of increased adhesiveness to the skin thus facilitating the dermal delivery. The interest towards this technology for dermal application has been raising since 2007, when the first anti–aging and skin–protective cosmetic products based on nanosuspensions of poorly soluble antioxidants rut in and hesperidin have been introduced to the market.57
Rutin nano crystals were produced by a combination process of low energy pearl milling followed by high pressure homogenization. When applied to the skin of human volunteers, nanosuspension with 5% rutin as non–dissolved nanocrystals showed a 500–fold higher antioxidant activity compared with a 5%solution of a water soluble rutin–glycoside derivative. Due to the higher solubility of active rutin as a nanocrystal, there is an increased concentration gradient between dermal formulation and skin, which leads to a higher penetration when compared to large drug crystals. Rutin penetrating into the skin is immediately replaced by fast dissolving molecules from the nanocrystals. Moreover, compared to the hydrophilic rutin–glycoside, the original lipophilic molecule rutin can better penetrate the skin where it is believed to have a higher affinity to the sites of action than the synthetically modified derivative.57. As a consequence of these positive outcomes, several researches on nanosuspensions of antioxidant molecules have been reported in literature since 2007.
First, Mishra produced nanocrystals of hesperidin, the aglycone of hesperidin, which not only has anti oxidative but also anti–inflammatory properties and, therefore, is thought to be a very effective anti–aging compound. As, there is no difference in the procedure for producing nanocrystals for oral, intravenous or dermal application. The only difference in the formulations is the choice of the stabilizers. Hence, in this study nanosuspensions of hesperetin were produced by high–pressure homogenization employing four different stabilizers suitable for dermal use such as Poloxamer 188, Inutec SP1, Tween 80 and Plantacare2000, possessing different mechanisms of stabilization. Interestingly, as predicted from the zeta potential measurements, nano suspensions stabilized by Inutec and Plantac were stable with no change in mean diameter size. Slight increases in size were found for nanosuspensions stabilized by Poloxamer and Tween, which is not considered to impair their use in dermal formulations.
In 2011, Kobierski chose the anti–oxidant resveratrol as an ideal candidate to be used as nanocrystal in dermal cosmetic formulations such as creams, lotions and gels. Since preservation is essential for dermal formulations, resveratrol nanosuspensions were produced by a high pressure homogenization process and the effects of preservatives on physical stability were monitored as a function of cycle numbers.
The same year, Mitri prepared a lutein nanosuspension by means of high pressure homogenization to enhance dissolution velocity and saturation solubility, which are major factors determining oral bioavailability and skin penetration. Lutein is a well–known antioxidant and anti–free radical used in cosmetic, nutraceutical industry with potential application in pharmaceutics as supportive antioxidant. The main results from this study indicated that lutein nanocrystals significantly increased drug saturation solubility as well as permeation through cellulose nitrate membranes, compared to coarse powder. Moreover, no permeation through pig ear skin occurred, which supports the fact that lutein rather penetrates the skin and remains there acting as antioxidant.
A solid in oil nanosuspension of diclofenac sodium was the first pharmaceutical formulations in which nanosuspension technology was applied to topical application of a poorly soluble therapeutic agent. In this study, Piao successfully dispersed Diclofenac sodium into isopropylmiristate (oil phase) as a nanosized suspension via complex formation with sucrose ester surfactant. The drug flux across the Yucatan micro pig skin model was increased up to approximately 4 fold compared with a coarse oil suspension used as control. In a more recent paper, nanosuspension gel formulations were studied as suitable formulation approach for improving the skin permeability of a poorly soluble molecule such as ibuprofen, a non–steroidal anti–inflammatory drug used for the treatment of acute and chronic arthritic conditions. Since the ibuprofen oral administration can cause gastric mucosal damage which may result in ulceration, its topical delivery can overcome many side effects such as gastric complications. In this study, tocopherylpolyethyleneglycol1000 succinate (TPGS) and hydroxylpropylmethylcellulose (HPMC) were used as the basic components in nanoparticle ibuprofen formulations. TPGS was used to enhance the drug permeability (tested in both porcine and human skin) and to stabilize the system by hydrophobic interactions, while HPMC was used as a steric stabilizer to inhibit crystal growth of the drug in the formulation. Results of experiments clearly indicated that the overall skin permeation enhancement process strongly depended on the solubilizer and the particle size of the drug crystals. These factors resulted in higher drug release due to the formation of supersaturated solution around the crystals and thus a high concentration gradient was produced between the drug crystals and skin surface. It can be concluded that a number of factors including the particle size of the drug crystals, surface properties of the carrier, interaction of drug molecule with the stabilizer needed to be considered while designing a suitable dermal formulation for poorly soluble compounds.
In 2013, Lai tested nanosuspensions prepared by simple precipitation method as a tool to improve cutaneous targeting and photo stability of tretinoin, poor water soluble and instable molecule, commercially formulated in cream and gel forms for the treatment of acne vulgaris. Drug skin permeation and deposition were studied in vitro by diffusion experiments through new born pig skin while TRA photo stability was investigated by irradiating samples with UV light. As an appropriate comparison, anO/W nanoemulsion was also prepared and tested. Indeed, nanoemulsions have shown to be particularly useful as vehicle for dermal and transdermal delivery of hydrophobic compounds for pharmaceutical, cosmetic, and chemical industry applications. During 8 hyper cutaneous experiments, the nanosuspension was able to localize the drug into the pig skin with a very low transdermal drug delivery (which is responsible for the systemic side effects of this drug), whereas the nanoemulsion greatly improved drug permeation.
Therefore, this work demonstrated the high potentiality of nanosuspensions in dermal drug delivery. It is worth noticing that nanosuspensions are almost exclusively composed of the drug nanoparticles with small amounts of biocompatible and safe surfactants, such as the soy lecithin used in this work. Although only few toxicological data are available at the moment, no side effects are known or expected from nanocrystal topically applied. Indeed, as suggested by Muller et al.2 each solid macro/micro–particle applied on the skin will convert into nanocrystals during its dissolution process and, up to now, no intolerability has been reported.2 As already reported above, also in this research paper authors justified the increased drug dermal delivery as a consequence of increased concentration gradient between the dermal formulation and the skin. More specifically, they claimed that finely divided and uniformly suspended tretinoin nanocrystals possess an increased dissolution rate due to their large surface area and increased saturation solubility. Solid drug dissolves in the vehicle, diffuses through the vehicle to the skin, and establishes local phase equilibrium with the outer layer of skin and finally the larger concentration gradient penetrates the skin forming a depot in the lipophilic SC from which it diffuses.
Furthermore, the topical application of tretinoin nanocrystals has the advantage of increasing the drug photo stability, in comparison with the control nano emulsion. In the last two years, the application of nanocrystal technology has been extended to medium soluble compounds and a new mechanism by which nanocrystals can improve dermal delivery has been proposed, in addition to the simple increase of concentration gradient between formulation and skin. This second mechanism involves the hair follicles.
Nanocrystals with an appropriate size (approximately 700nm) can accumulate into these shunts, which act as a depot from which the drug can diffuse into the surrounding cells for prolonged release. In a novel interesting paper, Zhai produced nanocrystals with the aim of increasing the skin penetration of a medium soluble active such as caffeine. Moreover, they developed a specific production process, namely a low energy milling in low dielectric constant dispersion media and a selected stabilizer, which allowed to overcome crystal growth and fiber formation caused by super saturation and recrystallization effects, typical with medium soluble compounds. The novel concept introduced by Zhai, consisted of formulating nanocrystals from medium soluble actives, and applying a dermal formulation containing additional nanocrystals, which should act as fast dissolving depot, increase saturation solubility and accumulate in the hair follicles, to further enhance skin penetration. For this purpose, they produced caffeine nanocrystals with varied size ranging from 660 nm (optimal for hair follicle accumulation) to 250 nm (optimal for fast dissolution), by pear milling process inethanol/propylene glycol 3: 7 with 2% carbopol.
Nanosuspensions appear to be unique & yet commercially viable approach to combating problems such as poor bioavailability that are associated with the delivery of hydrophobic drugs, including those that are poorly soluble in aqueous as well as organic media. The dissolution problems of poorly water soluble drugs have been largely solved to improve drug absorption & bioavailability. Drug nanoparticles can also be incorporated into water free ointments and creams, which have an increased saturation solubility and enhanced diffusion of drug into the skin. To take advantage of nanosuspension drug delivery, simple formulation technologies & variety applications, nanosuspensions will continue to be interest as mucosal formulations & topical administration develop in the future.
None.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.