Journal of
eISSN: 2377-4282


Research Article Volume 3 Issue 6
1Departamento de Ciencias Quimico-Biologicas, Universidad de las Americas Puebla, Mexico
2Instituto de Fisica, Benemerita Universidad Autonoma de Puebla, Mexico
3Department of Chemistry and Biochemistry, Texas Christian University, USA
Correspondence: Miguel A Mendez-Rojas, Laboratorio de NanoBioInorganica, Departamento de Ciencias Quimico-Biologicas, Universidad de las Americas Puebla, ExHda. Sta. Catarina Martir s/n, San Andres Cholula, 72820, Puebla, Mexico, Tel 52-222-2292607
Received: March 11, 2016 | Published: July 1, 2016
Citation: Alan VGB, Isabel CPL, Eugenia MM, Roberto GR, Coffer JL, et al (2016) Hollow Magnetic Iron Oxide Nanoparticles as Sodium Meclofenamate Drug Delivery Systems. J Nanomed Res 3(6): 00071. DOI: 10.15406/jnmr.2016.03.00071
Sodium meclofenamate is a widely used anti-inflammatory drug which has to be administered periodically to keep their physiologically active dose in serum. The development of nanostructured drug delivery systems may be useful for sustained release of the molecule for longer time periods. Magnetic hollow iron oxide nanocapsules were successfully synthesized by the solvothermal decomposition of an urea/FeCl3∙6H2O complex, as metal precursor, and Ethylene Glycol (EG) as solvent; the reaction was carried out at 200°C. The morphology and physical properties of the nanocapsules were characterized using X-Ray Diffraction (XRD), Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), Infrared Spectroscopy (IR), and Energy Dispersive X-Ray Spectroscopy (EDS). UV-Vis was used to determine the adsorption and release capacity of Sodium Meclofenamate (SMF). Spherical Hollow Magnetic Nanocapsules (HMNCs) 220-230 nm in size were obtained; they were able to load up to 18% of total available SMF in solution after 18 hours and then release efficiently and steadily 45% of it after 6 hours in aqueous suspensions.
Keywords: Magnetic nanoparticles, Hollow nanocapsules, Sodium meclofenamate, Drug delivery
ATR, Attenuated Total Reflectance; DMSO, Dimethylsulfoxide; DMF, Dimethylformamide; EDS, Energy Dispersive X-Ray Spectroscopy; EG, Ethylene Glycol; FTIR, Fourier Transforms Infrared; MHNCs, Magnetic Hollow Nanocapsules; SEM, Scanning Electron Microscopy; SMF, Sodium Meclofenamate; TEM, Transmission Electron Microscopy; UV, Ultraviolet; XRD, X-ray Diffraction
The exploration of nanostructured platforms for drug delivery has experimented a tremendous growth in recent years due to their promising potential to control the precise amount of pharmacological active molecules liberated along a specific time.1-2 Different nanostructured materials have been prepared and their performance as reservoirs for drug transport evaluated. Current limitations on the field include limited effective targeting, potential toxicity, suboptimal bioavailability, low transportation efficiency, uncontrolled release, among others. Several promising and versatile nanoscale systems, such as nano gels, carbon nanotubes, dendrimers, nanocapsules and nanoparticles have been extensively evaluated.3 Among the different bioactive molecules they can be able to carry we may find peptides, proteins, nucleic acids (RNA and DNA), natural and synthetic oligonucleotides, anticancerigen drugs, gene fragments, antibiotics, antioxidants, vitamins and many more.4 These nanostructured drug delivery systems try to overcome some of the main limitations associated with pharmacological therapeutics such as short plasma half-life, poor stability and immunogenicity, as well as to try to maximize the therapeutic activity while minimizing the toxicity. As smaller carriers have larger surface area-to-volume ratios, increased solubility is expected besides the potential to transport large doses per particle of pharmacological active molecules.5
Hollow nanoparticles may play an important role in the development of nanoencapsulation systems. In particular, magnetic hollow nano systems may be of interest as they are able to be manipulated by an external magnetic field, concentrating them in a specific point of the body or moving them along a specific biological path toward a target site .6-10 Due to their inherent magnetic properties, they can also be used for both diagnostics and therapeutics, a dual field of great attractiveness to the pharmaceutical industry known today as “theranostics”. They can be obtained through different chemical and physical approaches, being the solvothermal method a simple and easy procedure to prepare hollow nanoparticles with good control of size and morphology.11
Nonsteroidal anti-inflammatory drugs, such as aspirin, sodium diclofenac, piroxicam, tenoxicam, ibuprofen and sodium meclofenamate are widely sold and consumed around the world due to their effectiveness, low prices and availability. 12-17 In particular, sodium meclofenamate (pKa = 4.39, soluble in water, ethanol, DMSO and DMF), is a very useful drug for the symptomatic treatment of moderate pain, several forms of arthritis, dysmenorrhea and menorrhagia (Figure 1).18 Moreover, it is currently being studied for its antitumor effects in murine models which may confer this drug a promising anti-neoplastic potential.19,20 Because of its increased risk of serious gastrointestinal events i.e. bleeding and ulceration, it is recommended to use the lowest effective dosage and shortest duration of therapy and to avoid its administration on empty stomach even when food decreases rate and extent of absorption.18 Based on this, a novel meclofenamate delivery approach may be useful.
We have recently explored the preparation and surface chemical modification of metal oxide nanoparticles for biomedical and nutritional applications.21-23 in particular, we reported the evaluation of biocompatible magnetic nanoparticles for controlled drug delivery of vitamin E into Si Ha cancer cells to induce apoptosis.24 In this work, Magnetic Hollow Nanocapsules (MHNCs) were prepared by a solvothermal method and their ability to load and release Sodium Meclofenamate (SMF) was evaluated.
Material
All chemicals were purchased from Sigma Aldrich (Toluca, Mexico) and used as delivered without further purification. Iron (III) chloride hexahydrated (FeCl3∙6H2O), urea, Ethylene Glycol (EG) and Sodium Meclofenamate (SMF), were reagent grade. Fourier Transform Infrared (FTIR) spectra were recorded as grinded solids on a Varian Scimitar FTIR-800 spectrophotometer equipped with an ATR detector and recorded in the region 4000-400 cm-1. Surface morphology and composition of the nanoparticles were analyzed using a Vega II Scanning Electron Microscope (SEM, Tescan, Czech Republic) instrument, equipped with an Energy Dispersive X-Ray Spectrometer (EDS) detector. Transmission Electron Microscope (TEM) images were obtained on a JEOL J-2100 electron microscope at an acceleration voltage of 200 kV. TEM samples were prepared by depositing one drop a dilute suspension of the sample in water on a carbon-coated copper grid and allowing the solvent to evaporate at room temperature. Powder X-ray Diffraction (XRD) measurements were performed on a PANalytical Empyrean diffractometer on grinded powders in a quartz sample holder using Cu K∝1 line source (λ = 1.54018 A) in 2θ mode (measuring interval: 10 to 80°); step scan = 0.02; step time = 0.6 sec. The amount of loaded or released Sodium Meclofenamate (SMF) was calculated from the corresponding UV-visible absorbance, using a calibration curve previously measured in a Cary 100 UV-visible spectrophotometer.
Synthesis of [Fe(urea)6]Cl3
[Fe(urea)6]Cl3 was prepared by the solid state reaction of FeCl3.6H2O and urea at room temperature, following the same procedure reported by Guan and coworkers .25 Typically, 4.36 g of urea and 3.24 g of FeCl3.6H20 were grinded finely in an agate mortar for 1 h. After mixing and grinding for 2 more hours, a yellow powder was obtained. Chemical identity was confirmed by FTIR spectroscopy.
Preparation of Iron Oxide Magnetic Hollow Nanocapsules
The Iron oxide magnetic hollow nanocapsules (MHNCs) were prepared through a modified solvothermal method. Typically, 0.50 g (0.95 mmol) of the [Fe(urea)6]Cl3 complex were dissolved in 20.0 g of EG (53.07 mmol) in a sealed glass ampule and stirred vigorously at room temperature until a homogeneous solution was formed. This solution was introduced into the oven and heated at 200°C for 72 hours. After this time, heating was turned off and the system was cooled slowly until it reached room temperature. The product was washed with absolute ethanol to remove residual reagents and magnetically decanted with a permanent strong Nd magnet. Sample was characterized by SEM, EDS, TEM and DRX.
Loading of MHNCs with sodium meclofenamate
Sodium Meclofenamate (SMF) was incorporated into the MHNCs by dispersing 50 mg of MHNCs into 10 mL of an aqueous solution containing 25 µg of SMF (8.0 µM). The mixture was magnetically stirred for 3 minutes and then, without stirring, it was kept still during 24 hours at room temperature; the concentration of free SMF in solution was followed spectrophotometrically during that time. After 18 hours, 18% (4.5 µg of SMF) of the initially available SMF was loaded into the MHNCs, then the product was magnetically decanted, washed twice with a few drops of cold 2:1 ethanol: water solution to remove weakly adsorbed molecules of SMF on the surface of the MHNCs, dried at room temperature and stored in a freezer for further characterization.
The percent of loaded drug was calculated from the amount of SMF adsorbed into the MHNCs with respect to the initially available SMF concentration (equation 1):
In vitro drug release determination
50 mg of previously prepared, dried, SMF-loaded MHNCs were dispersed into 10 ml of pure distillated water (pH = 6.9) and gently stirred for homogeneity. The release of SMF into the medium was followed by UV-Vis spectrophotometry. After 6 hours, ~2 µg of SMF (almost 45% of the initially loaded SMF) were released, with the system reaching an equilibrium status without no further significant changes in the released-SMF concentration in solution after this time.
The percent of released SMF was calculated from the initial amount of SMF loaded into the MHNCs and the measured released-SMF in solution along the time, according with (equation 2):
Almost mono dispersed magnetic hollow nanocapsules (MHNCs) were obtained by a solvothermal reaction from the thermal decomposition of the [Fe(urea)6]Cl3 complex used as metal precursor. The infrared absorption spectra (FTIR) of the iron (III) urea product confirmed that the [Fe(urea)6]Cl3 complex was successfully obtained (Figure 2). A strong absorption band corresponding to a C=O stretching vibration appears at 1619 cm-1, while a band corresponding to a C-N stretching vibration is observed at 1487 cm-1. These bands are shifted from the typical values for free urea (1686, 1466 cm-1) as expected for an urea coordinated to a Fe3+ ion. Another medium band appears at 3210 cm-1, characteristic of a N-H stretching vibration, as well as a C-O stretching vibration at 1029 cm-1.
Once the iron metal complex hydrolyses the thermal decomposition of urea yields carbon dioxide microbubbles which are used as templates for the hollow nanocapsules formation. Ethylene glycol, a high boiling point solvent (196-198°C) played a key role in the preparation method as a dispersing medium as well as reducing agent and a stabilizing surfactant. The chemical reduction of Fe(III) under the experimental conditions to Fe(II) by EG and the condensation of these cations into a spinel-type structure (as confirmed by the XRD analysis), explain the formation of a highly crystalline phase of magnetite/maghemite after 24 hours of reaction, as shown in the diffractograms of (Figure 3). Comparison of the obtained XRD pattern to that of magnetite (JCPDS No. 79-1417) confirm that all diffraction peaks (220, 311, 400, 511, 440) corresponds to a face cubic centered (fcc) crystalline phase pattern, characteristic for Fe3O4, indicating that the MHNCs were successfully obtained. Due to the small amount (and poor crystallinity) of the loaded SMF, no attempts were made to analyze by XRD the SMF loaded MHNCs as the only expected diffraction peaks would be those corresponding to the MHNCs.
SEM analysis of the MHNCs clearly show that the products have a spherical morphology (Figure 4a). The particles are agglomerated and are uniform in size and shape. EDS chemical composition analyses of these particles (Fe, 72.8%; O, 27.2%) are in agreement with what is expected theoretically for magnetite (Fe, 72.4%; O, 27.6%). Average size of the hollow spherical particles is in the range from 220 to 230 nm. TEM images (Figure 4b-4d) indicate that the particles possess a porous shell, with a shell thickness of about 50-60 nm. Some of the analyzed MHNCs have an open structure, showing the empty inner cavity, clearly indicating than their surface is rough and non-uniform.
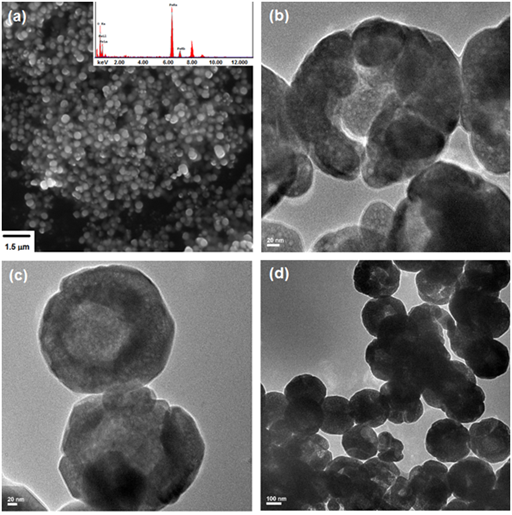
Figure 4 Scanning Electron Microscopy (SEM) (a) and Transmission Electron Microscopy (TEM) (b), (c) and (d) images of MHNCs.
As shown in the SEM and TEM analyses, these magnetic hollow nanocapsules have accessible inner surface, ready to be loaded and used for drug delivery and transport. Sodium Meclofenamate (SMF), a well-known anti-inflammatory agent, was loaded into the MHNCs by soaking them into an aqueous SMF solution. After 18 hours, the SMF-loaded MHNCs were removed from the solution by magnetic separation, washed twice to remove weakly adsorbed SMF and dried. SMF was successfully loaded into the MHNCs as indicated by the decreasing on the SMF concentration in the initial loading-solution (Figure 5a). A total of nearly 4.5 µg of SMF (18% respect to the initial amount of available SMF in solution) were loaded into the MHNCs. On the other hand, when the SMF-loaded MHNCs were re-dispersed in pure distillated water (pH 6.9), they started to immediately release free-SMF into the medium. After 6 hours, almost 2 µg of SMF were released (nearly 45% of the initially loaded SMF), reaching at this time an equilibrium among the concentration of free-SMF in solution, SMF loaded into the MHNCs and SMF solubility in water. Monitoring of the concentration of SMF after 6 hours do not show any significant change with respect to this equilibrium. Considering that the amount of MHNCs used for these studies (50 mg of pure MHNCs), this system is able to load up to 90 µg of SMF per gram of nanocarriers, and then release 40 µg of SMF per gram of nanocarriers.
As the maximum loading concentration of SMF in the MHNCs was found to be ~90 µg/g (about 18% of encapsulation efficiency, (Figure 5a), it may indicate that the affinity among the drug and the MHNCs at the studied conditions is not yet optimal; it may be possible to increase the amount of SMF loaded into the nanocapsules by changing the pH of the loading solution, which may generate local charges on the surface of the MHNCs, increasing the chances for electrostatic interactions among them and the drug. The In Vitro SMF release profile (Figure 5b) indicates that almost 45% of the transported drug (~ 2 µg) can be liberated after 6 hours, reaching an invariant concentration equilibrium after this time. It can be inferred from this behavior than for a dynamic system where the concentration of released drug decreases as it is consumed, a controlled, long time range may be reached, keeping without significant alterations the desired optimal pharmacological serum concentrations. Also, changes in the pH of the solution, temperature and SMF loaded concentration, as well on the size of the MHNCs may affect the kinetics of SMF releasing, helping to improve drug release.
Magnetic Hollow Nanocapsules (MHNCs), made of porous nanostructured magnetite (Fe3O4), have been successfully prepared. These MHNCs, with uniform spherical morphology and sizes in the range about 220-230 nm, have been evaluated as drug release systems for the controlled liberation of sodium meclofenamate (SMF) and their performance indicates that they are able to load about 18% of available drug after 18 hours, and release nearly 45% of contained molecule after just 6 hours, in aqueous suspensions. Further work on the determination of loading and release performance in physiological conditions (PBS solution, pH 7.2) has to be performed, in order to determine their potential utility As In Vivo drug delivery systems. In order to improve their biocompatibility and solubility in water, MHNCs may be further functionalized on their surface using biocompatible polymers such as polysaccharides, silica or PEG. Future studies on the ability of MHNCs to transport other pharmacological active molecules or to be used as Magnetic Resonance Imaging (MRI) contrast agents, are currently undergone.
None.

©2016 Alan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.