Journal of
eISSN: 2377-4282


Editorial Volume 5 Issue 2
1Department of Chemistry, Aligarh Muslim University, India
2Department of Saidla, Aligarh Muslim University, India
Correspondence: Rahman A, Department of Saidla (Unani Pharmacy), Aligarh Muslim University, Aligarh-202001, India
Received: February 23, 2017 | Published: March 3, 2017
Citation: Siddiqi KS, Rahman A, Tajuddin (2017) Formation of Ag/AgCl Nanoparticles. J Nanomed Res 5(2): 00108. DOI: 10.15406/jnmr.2017.05.00108
This is with reference to the article “Yeast- derived biosynthesis of silver/silver chloride nanoparticles and their anti proliferative activity against bacteria” RSC Advances Mateus Eugenio, Nathalia Müller, Susana Frasés, Rodrigo Almeida-Paes, Luís Maurício T. R. Lima, Leandro Lemgruber, Marcos Farina, Wanderley de Souza and Celso Sant'Anna; 2016, 6, 9893-9904, about the synthesis and some abntibacterial properties of Ag and Ag/AgCl nanoparticles (NPs) formed from 15 clonal yeast isolated from termite gut.
The authors isolated yeast from the gut of 10 termites (TERM) from the species Cornitermes cumulans. The isolates were grown in culture medium containing AgNO3. A colour change from pale yellow to brown was ascribed to the formation of AgNP after 7 days in seven isolates (Table 1). A similar change in colour was also observed when AgNO3 and the supernatant of dead cell culture of isolates were incubated at 30°C. surprisingly, it has also been ascribed to the formation of Ag/AgCl NPs (Figures 1A & 1C). Authors have suggested that it was due to extracellular formation of Ag/AgCl NPs. When extracellular and intracellular or dead and live bacterial cell culture yield the same result there does not seem to be any valid reason to believe that AgCl was also formed along with AgNPs. AgCl NPs can be formed only if there is any species that furnishes Cl- ions in solution. And there is no addition of any chloride salt which may precipitate Ag+ ions as AgCl NP according to the following reaction.
AgNO3 + NaCl → AgCl + NaNO3
|
Yeast Isolate |
Medium Color Change |
Uv-Vis Absorbance (AU) |
|
TERM73 (SNPP1) |
Dark brown |
2.75 |
|
TERM77 (SNPP2) |
Dark brown |
3.13 |
|
TERM78 |
Light brown |
1.68 |
|
TERM79 |
Light brown |
1.9 |
|
TERM82 |
Light brown |
1.74 |
|
TERM83 |
Light brown |
1.89 |
|
TERM89 |
Light brown |
1.77 |
Table 1 Screening of yeast isolates capable of producing Ag/AgCl-NPs
The authors of this paper have shown that formation of Ag/AgCl NPs by yeast isolates grown in the presence of AgNO3 was confirmed by an absorption in 350-450nm range in the UV-vis spectrum (Figures 1B & 1D). This is attributed to the excitation of surface plasmaon resonance in Ag NPs. However, it may not be due to AgCl resonance because the presence of AgCl is dubious. The change in colour cannot be taken as an evidence for the formation of AgCl NP because the absorbance observed at 410 and 415nm are due to Ag NPs.1,2 Generally, the absorption peaks noted in 400-465nm in UV-vis spectra have been attributed to the formation of AgNP in solutions.3,4 If the AgCl is formed even in traces, it would settle at the bottom of the container and slowly turn grey due to its exposure to sun light but not brown as has been observed by authors. However, AgCl is solid and its solubility in water is negligible because its solubility product is too low to furnish AgCl ions.
KspAgCl = 1.77x10-10 (At room temperature)
The presence of AgCl would have been confirmed by the addition of ammonia which dissolves AgCl to give a clear solution. It can be reconfirmed by the addition of nitric acid to the above solution which gives white precipitate of AgCl again, as shown below.
AgCl + 2NH4OH → Ag(NH3)2Cl + 2H2O
(Solid) (Soluble)
Ag(NH3)2Cl + 2HNO3 → AgCl + 2NH4NO3
(Soluble) (Solid)
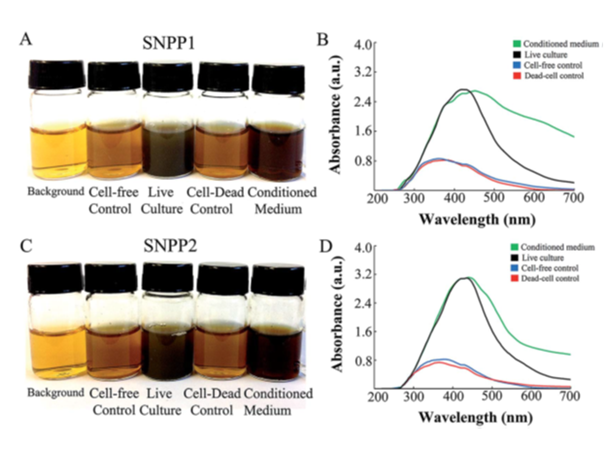
Figure 1 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
These confirmatory tests were not done to verify the presence of AgCl NPs nevertheless the authors are banking solely on the colour change which may only be due to the presence of silver NP. The authors have suggested that the absorption peaks appearing at 434 and 435 from NPs produced from two termites (TERM 73, 77) and AgNO3 are due to Ag/AgCl NPs. Further, heat killed yeast suspension containing same termites (TERM 73, 77) having dead strains of Candida lusitaniae and AgNO3 showed absorptions at 349 and 355 nm respectively. It has been suggested that reduction of Ag /AgCl NPs occurred which exhibited the above absorption peaks. It is still not clear as to what was the source of chloride ion that resulted in the formation of AgCl. Presence of chlorine has been inferred from SEM and EDS images which showed peaks for it. At this stage the presence of Cl cannot be denied but the source is unclear.
We have tested commercially available yeast for chloride ion. The supernatant of yeast solution in distilled water was incubated with 1mM solution of AgNO3 at room temperature (i) and (ii) also after heating at 70°C in two different sets. The precipitate was formed and a change in colour occurred but it did not dissolve in NH3. This precipitate may have been formed due to the formation of silver hydroxide but it is not AgCl because it did not dissolve in ammonia.
None of the references.5-7 cited by the authors of the above paper at serial number 1,12,13 are related to Ag/AgCl formation. They are not relevant and give only the formation of silver NPs. The formation of Ag/AgCl- NP from AgNO3 in absence of chloride ions is highly improbable.
None.
None.

©2017 Siddiqi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.