Journal of
eISSN: 2377-4282


Research Article Volume 4 Issue 3
1Materials Department, Shahrekord University, Iran
2Biology Department, Isfahan University, Iran
Correspondence: A Doostmohammadi, Materials Department, Shahrekord University, Engineering Faculty, Shahrekord, Iran, Tel 98 (913) 326 6632, Fax 98 (38) 32324438
Received: October 31, 2016 | Published: November 15, 2016
Citation: Doostmohammadi A, Esmaeili F, Katouli SN (2016) Fabrication of Chitosan/Poly (vinyl alcohol)/Carbon Nanotube/Bioactive Glass Nanocomposite Scaffolds for Neural Tissue Engineering. J Nanomed Res 4(3): 00088. DOI: 10.15406/jnmr.2016.04.00088
The purpose of this study was to fabricate chitosan electro spinning nanofiber (CS)/poly (vinyl alcohol) (PVA) containing carbon nanotube (CNT), and bioactive glass nanoparticles (BG) (5 and 10 wt %) for neural tissue engineering applications. The Morphology and structure of electro spun composite nanofiber were characterized using scanning electron microscopy (SEM) and tensile strength of the electro spun composite fibre was measured. Also embryonic carcinoma stem cells (P19) were cultured on the electro spun scaffold. The results showed that the existence of carbon nanotube (CNT) and bioactive glass (BG) doesn’t appreciably affect the morphology of chitosan nanofibers (CS) and poly (vinyl alcohol) (PVA). The maximum tensile strength (7.9 Mpa) was observed in the composite samples of bioactive glass nanoparticles (BG) with 5 wt%. In addition, proliferation and bioactivity of embryonic carcinoma stem cells (P19), in contact with scaffolds containing bioactive glass nanoparticles, were more than scaffolds with no glass.
Keywords:Nanofibers, Composite scaffold, Carbon nanotube, Bioactive, Glass nanoparticles, Electro spinning, Neural tissue engineering
Tissue engineering in medical engineering has been always raised and expanded for the repair and replacement of damaged or destroyed tissues and organs operations. Nerve regeneration is a precious treatment for human health because it is directly related to the quality of human life. It is quite clear that human central nervous system (CNS) in adults is unable to repair itself after being damaged or injured.1,2 Recently, tissue engineering has offered a new therapy as an alternative for the traditional method of graft including the use of polymeric or composite biomaterials with or without cells. Polymers can be used as scaffolds for providing cell adhesion, maintenance, and differentiation without making the delay in cell differentiation.3 Poly (vinyl alcohol) (PVA) is known as a biocompatible and hydrophilic polymer with proper chemical and thermal stability and also excellent tensile strength and flexibility.4,5 Chitosan is a linear polymer with a polysaccharide structure and obtained from hydrolyzing natural polymer of chitin.6 Chitosan is also one of the biodegradable and biocompatible polymers with human body and has certain features appropriate for many fields such as biological, medical, drug delivery, and gene therapy.7 Chitosan polymer composites and poly (vinyl alcohol) have been used in neural tissue engineering.1,4,7 The proper rate of degradation, acceptable mechanical properties, and high spinning capability are the main reasons for using these polymers in fabricating neural tissue engineering scaffolds.5,6 The most important requirement of a tissue engineering scaffold is having proper mechanical properties to be able to tolerate dynamic and static stress of body fluid environment. Neural tissue engineering scaffolds are not exempt from this. Carbon nanotubes have an important and special place among materials with high potential to be used in micro/nano systems. Notable properties of carbon nanotubes such as high conductivity, mechanical strength, low density, and high stability have led them to be considered by the researchers in recent years. Of these, high conductivity and good mechanical strength have expanded their use in neural tissue engineering scaffolds. In recent years, researchers have mentioned the use of carbon nanotubes and their roles in neural tissue engineering scaffolds as a part of conductors and directors of neural signals.1 Scientists have found that nonwoven single-walled carbon nanotubes have more scaffold properties than any other types. So, cell adhesion and proliferation ability increase significantly.8,9 This finding opens a new way of using nanotubes in repairing cell damages. Tight connections of osteoblasts to nanotubes scaffolds formed during the cultivation have been approved before.10-12 Bio ceramics are known and famous groups among biomaterials. High corrosion resistance, biocompatibility, and proper biological properties are major advantages of these materials than metal and polymer ones.13 Among all bioactive materials, the best bioactive treatment belongs to the bioactive glass containing a group of glass compounds which bond to the tissue in a short time. The first reports of in vitro studies about bioactive glasses published in 1971.14 Integrated pieces of bioactive glasses (45S5 Bio glass) were placed in tibia of a rat and not only didn’t make any inflammatory effect, but also created a substantial connection with the surrounding bone tissue.15 The capability of bioactive glasses in repairing soft tissue and their potential effect on tissues such as neural tissues are mentioned in some papers.16,17 However, no reports in this field have been published yet.
Nanofibers are suitable choices for the role of extracellular matrix (ECM). In recent years, electrospun nanofibers are of great interests because of the proximity of their structure to the fibrous tissue and extracellular matrix structure and also high effective level of cells adhesion and growth. Studies have been expanded on these scaffolds. Electro spinning is the most widely method used in fabricating nanofibers with no limitation for all types of polymers.18 It is a simple method known as one of the best in fabricating nanofibers. This method which was first reported by Formhal controls fabricating polymer fibers on our target object by the use of electric field.19,20 In this research, fabrication of chitosan/poly (vinyl alcohol)/carbon nanotube/ bioactive glass nanocomposites with improved biological and mechanical properties has been considered for neural tissue engineering. Bioactive glass nanoparticles were used due to their ability in repairing soft tissues.13,17 and their increasing effect on the scaffolds’ mechanical properties.
Preparation of precursor solutions of scaffolds
Preparation of PVA: A certain amount of distilled water was put on the surface of the stirrer to be reached at 80 °C. Then poly (vinyl alcohol) (PVA) powder (Hydrolyzed, Merck, 99%, 72000 Mw) was added to the water. This solution was placed on the magnetic stirrer until a homogeneous solution was achieved. The stirring time depended on the time the solution needed to be homogeneous and it took 1-3h.
Preparation of CS/PVA/CNT: First, 0.05 gr of chitosan (Sigma 28191 from Crab Shell) was stirred for 10 min with an ultrasonic stirrer. It was then put on a magnetic stirrer for 10 h to prepare a uniform solution. After the preparation of solution, the prepared PVA was added with the ration of 25/75 (PVA/CS) and stirred for 1 h. Then 0.0005 gr of carbon nanotube (CNT) (Supplied by Neutrino, 98% Purity) was stirred in acetic acid solution (3%) for 20 min with an ultrasonic stirrer and finally added to the CS/PVA solution.
Preparation of CS/PVA/CNT/BG: The process of preparing this solution was as the same as the previous one except that after stirring chitosan, 0.0025 and 0.005 gm of bioactive glass nanoparticles (5 and 10 wt %) was added to the solution, respectively. Then the solution was stirred completely for 10 min with an ultrasonic stirrer. Finally, the PVA solution was added to the main solution.
Preparation of scaffolds
Electro spinning process was used to fabricate the scaffolds. After the preparation of spinning solution, each intended solution was transferred to 2 ml or 5 ml syringes. Then, syringes were put in the electro spinning machine. Several tests were performed to find proper parameters in order to obtain desired nanofiber with the lowest possible bead. After that, the distance between tips of syringes to the collectors was chosen 11-12 cm with the solution jet exit speed of 0.4 ml/h from each syringe. The higher speed caused bead and the lower one caused syringe obstruction due to the decreased exit speed of the solution. 2700-2800 rpm of collector was used to achieve appropriate, parallel, and coaxial nanofiber. By applying a positive voltage of 7.7 and a negative voltage of 3.2 kV, the intended solution was put in an electro spinning machine for 3-4 h until the nanofiber be spun on the aluminium foil. The spun nanofiber was kept at room temperature for 2-3 days until the existence solvent was evaporated. After that, the nanofiber was separated from the aluminium foil. The process of cross-linking was performed by the Glutaraldehyde vapour (GA, Merck, 25%) in order to increase the consistency and mechanical strength of CS/PVA/CNT and CS/PVA/CNT/BG nanocomposites. For this purpose, 10 ml of GA was mixed with 40 ml of ethanol in order to obtain GA solution of 5 wt%. The nanofibers were exposed to this solution vapour and kept in the incubator.
Evaluation of CS/PVA/CNT and CS/PVA/CNT/BG nanofibers by FESEM: Field emission scanning electron microscopy (FESEM, MIRA3 TESCAN) was used to evaluate the scaffolds and study the structure and the diameter of spun fibres. After electro spinning of solutions and performing cross-linking, some cross-linked and non-cross-linked fibers were observed by FESEM. A small piece of scaffold was covered by gold and put on the sample holder
Mechanical properties evaluation: Tensile strength of the samples was measured according to the ASTM D638 standard. Samples were prepared with 0.01-0.02 mm width for CS/PVA/CNT and CS/PVA/CNT/BG scaffolds and tensile strength was determined with a mechanical testing machine (zwick-1446) at a crosshead speed of 5 mm/min.
Cell viability test: Cell viability test was performed according to the ISO 10993-5 standard. Sterilizing scaffold samples was carried out by being immersed in ethanol (70%). Then, samples of nanocomposite scaffold were immersed in DMEM culture media. Embryonal carcinoma stem cells (P19) were used in this study. These cells were prepared and proliferated from the cell bank at the Pasteur institute of Iran, Tehran. With the initial density of 5000 cells/well and in a 24-well plate (TCPS), cells were exposed to the culture medium including samples. For various times (0, 24, 72, and 96 h) after the culturing, MTT test was carried out to evaluate cells viability. At each time interval, after draining the medium and being washed by PBS, about 400 micro litres of medium along with 40 micro litres of the MTT solution (5 mg/ml) were added to the cultivation medium. Then it was kept in the incubator for 4 h at 37° C. The medium was then evacuated slowly. In order to dissolve the formazan, 200 microlitres of dimethyl sulfoxide (DMSO) (Dimethylsulfoxide, Merck) was added to the cultivation medium. The optical density was read spectrophotometrically at a wave length of 570 nm. The similar method was performed for stem cells in the culture medium with no scaffold samples as a positive control sample. All experiments were conducted at least for n=3. All of the data are expressed as mean ± SD. One-way analysis of variance (ANOVA) was used to compare results. A p-value of less than 0.05 was considered statistically significant.
Stem cells morphology and cell attachment on the scaffolds: MTT test was carried out to study the morphology of stem cells and their attachment and proliferation on different scaffolds. Scaffold samples were sterilized in 70 wt% ethanol and cells were washed in a 24-well plate (TCPS) with the initial density of 5000 cells/well with glycine and PBS and then were exposed to the culture medium including samples. For studying the growth and proliferation of cells and in order to study an adhesion effect of scaffolds in the first 24 h, after 3 days of cells culturing the culture medium was changed with DMEM solution containing 10% MTT and kept in the incubator for 4 days. Then the medium was evacuated slowly and in order to dissolve the formazan, 150 micro litres of dimethyl sulfoxide (DMSO) (Dimethylsulfoxide, Merck) was added to the cultivation medium. Cell carrier scaffolds were fixed in ethanol and were observed and evaluated by the field emission scanning electron microscopy (FESEM, MIRA3 TESCAN).
Evaluation of CS/PVA/CNT and CS/PVA/CNT/BG nanofiber by FESEM
Figures 1& 2 represent FESEM images of cross-linked and non-cross-linked sample of CS/PVA/CNT electro spun composite scaffold. Nanometre sized spun fibre can be seen well in FESEM images. Fiber diameter is varied from 80 to 250 nm. The morphology of cross-linked fiber confirms the cohesion of the final structure. This shows the necessity of cross-linking for reaching the maximum mechanical properties. Figures 3 & 4 also represent FESEM images of cross linked and non-cross linked sample of CS/PVA/CNT/BG electro spun composite scaffold. The results of FESEM show steady and uniform structures of CS/PVA/CNT and CS/PVA/CNT/BG nanocomposites with no bead but approximately the same diameter. These results confirm that existing polymer chains in both composites were entangled well with each other to create a uniform combination in all composite components. This fact confirms the absence of beads in nanofibers. According to the FESEM images, the morphology of nanofibers has not been changed significantly after cross-linking with glutaraldehyde vapour and also adding bioactive glass except the slightly increase in cross-linking nanofiber diameter. Increasing fiber diameter after the cross-linking step was probably due to the swelling of the nanofiber during the cross-linking process of GA vapour.21,22 It can be concluded that after the cross-linking step, the nanofibers porosity has been decreased. This behaviour was probably due to the increase in nanofibers diameter after this step which caused reducing the volume of the cavity of the fiber structure.21
Mechanical properties evaluation
Figure 5 shows the stress-strain curves of composites. The change in tensile strength of the samples by adding different percentages of bioactive glass nanoparticles is well observed. The existence of bioactive glass nanoparticles in the scaffold structure increases the biological activity of the scaffold on one hand and changes its mechanical properties on the other hand. As the results suggest, in the presence of glass particles, the flexibility of the scaffold has been decreased a little while its tensile strength increased. The effect of glass nanoparticles is various with different amounts. It can be concluded from studying and comparing the graphs of mechanical properties test of CS/PVA/CNT/BG nanocomposites (5 and 10 wt%) that bioactive glass composite with 5 wt% has better tensile strength than that of 10 wt% so that the mechanical properties of the scaffold will be decreased at values higher than 5 wt%. Therefore, it can be concluded that 5 wt% of a bioactive glass nanoparticles is an optimum and desirable composition. However, the presence of these particles in the scaffold structure along with creating bioactivity properties affects slightly the mechanical properties.
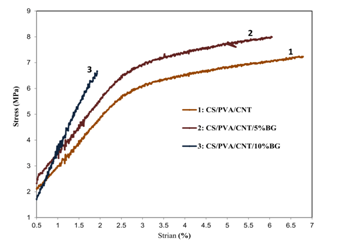
Figure 5 Stress-strain curves of (a) cross linked electrospun CS/PVA/CNT, (b) CS/PVA/CNT/5% BG, and (c) CS/PVA/CNT/10%BG nanofibers.
The decrease in mechanical properties by increasing the weight percentage of BG over 5 wt% is probably due to heterogeneous distribution of bioactive glass nanoparticles. It can be concluded that there is a threshold limit value for the weight percentage of bioactive glass nanoparticles in polymeric field until its mechanical properties be optimized. The amount more than this threshold limit value not only doesn’t reinforce polymeric field but also performs as a fault and harmful factor and decreases its mechanical properties. It is more likely that this happens due to the agglomeration of particles in high percentages which prevents suitable interaction between the polymer and bioactive glass nanoparticles and consequently power transmission doesn’t take place in nanoparticles.21 With different purposes, many studies have done on the composites of CNT and BG polymers in recent years and these composites have been always considered by the researchers.
By studying nanoparticles and bioactive glass nanoparticles, Boccaccini et al. 22 & Ruhani Esfahani.23 concluded that nanoparticles due to their high special surface increase the solubility characteristics of the particles. The higher amounts of glass nanoparticles in the polymeric matrix increase mechanical and biological properties of the composite. By studying polycaprolactone composite and bioactive glass nanoparticles, Tamjid et al.24 showed that bioactive glass micro particles indicate lower bioactivity, elasticity modulus, and mechanical properties compared to bioactive glass nanoparticles. Haji Ali et al.25 showed that there is a suitable relationship between the polymer matrix and bioactive glass nanoparticles which increases their mechanical and biological properties. Their studies also showed that bioactive glass nanoparticles, compared to the particles of materials with micro-size particles, have a considerable impact even in low value. All of these studies and researches show that adding bioactive glass nanoparticles into the polymeric composition increases mechanical properties of the scaffold. This result has been also proved in this research.
Effect of nanocomposite scaffold on the stem cells growth and proliferation (MTT assay)
Figure 6 shows the results of MTT test and the stem cells growth and proliferation in contact with CS/PVA/CNT and CS/PVA/CNT/BG nanocomposites. At the first glance it can be concluded that increasing the growth and proliferation of cells will be clearly seen in the control and both composite samples over the time. Although there was no significant difference between the numbers of cells in all samples in the first 3 days (Figure 6), the direct effect of CS/PVA/CNT and CS/PVA/CNT/BG nanocomposite scaffolds on the growth and proliferation of cells was clearly visible in the fourth day. After 4 days, stem cells growth and proliferation on CS/PVA/CNT scaffold was more than the control sample (p< 0.05). Providing a proper environment for the growth and proliferation of cells is the most important and effective cause of increasing cells growth in the presence of the scaffold.
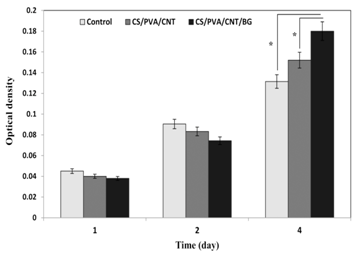
Figure 6 Comparison of the results of embryonic stem cells growth on control and CS/PVA/CNT and CS/PVA/CNT/BG nanocomposites samples at various incubation times. Average bioactivity cell (optical density) and standard deviation is shown at various times (p-value<0.05, n=3). Sign * indicates a significant difference between two groups.
An interesting result of this research is increasing the stem cell growth and viability on the scaffolds containing bioactive glass nanoparticles. The results show as well that in contact with CS/PVA/CNT/BG nanocomposite scaffold, proliferation and viability of stem cells are also more than CS/PVA/CNT nanocomposite scaffold. This superiority is due to the presence of bioactive glass nanoparticles in the composite scaffold. Increasing the cell growth in contact with bioactive glass nanoparticles has been also shown in previous researches.13,16,17,23-25
Mottaghitalab introduced chitosan/poly (vinyl alcohol)/carbon nanotube for neural tissue engineering. The human brain natural neural cells growth on prepared scaffolds was also studied and confirmed.26 After that, Liao et al.20 fabricated CS/PVA/CNT composite scaffolds through an electro spinning method. They revealed that L929 cells show appropriate response and reaction to the scaffold’s field and cell proliferation on CNT scaffolds is higher that scaffolds with no nanotube. This study confirmed the above results and suggested that the existence of bioactive glass nanoparticles on the scaffold increases the tensile strength of the scaffolds and cells growth and proliferation ability. This existence can increase the biological activity of the scaffold on one hand and change its mechanical properties on the other hand. As the results showed, the scaffold’s flexibility has been decreased a little in the presence of glass particles and its tensile strength increased. The effect of glass nanoparticles will be different in various amounts. However, the existence of these particles in the scaffold’s structure creates bioactivity property and affects a little on the mechanical properties. This result is corresponded with the achieved results of other researchers.23,25
Studying scaffolds biocompatibility with the embryonic carcinoma stem cells (P19) is very important due to the fact that in many cases neural tissue engineering scaffolds are used in places with no neural cells and neural primary and stem cells need to be grown and proliferated to be differentiated into neural cells in the next steps.
Adhesion of stem cells to the scaffold
The morphology of stem cells on nanocomposite scaffolds has been shown in Figures 7a & 7b. FESEM images show the spreading of cells on the scaffold. Cells spreading especially on the BG-containing scaffold are clearly obvious (Figure 7b). These results show that prepared scaffolds provided an appropriate environment for cells adhesion and proliferation. The final results of this study showed that CS/PVA/CNT/BG nano composite scaffold has both mechanical and biological properties and provides a suitable environment for stem and neural cells adhesion, growth and proliferation. The existence of CNT promotes mechanical properties and provides a necessary conductivity for the body of the scaffold.20 Therefore, it facilitates the possibility of neural cells connection. Bioactive glass nanoparticles increase the scaffold’s strength and also promote its biological properties. So, CS/PVA/CNT/BG nanocomposite scaffold is potentially an appropriate option to be used in neural tissue engineering.
In this study, CS/PVA polymeric nanofibers were fabricated through electro spinning method. CNT nanotubes and bioactive glass nanoparticles were used as enhancement components to improve biological and mechanical properties of nanocomposite. SEM images confirmed obtaining composite nanofiber. They also showed that composite nanofiber with bioactive glass nanoparticles of 5 wt% had higher tensile strength. Proliferation and bioactivity of embryonic carcinoma stem cells (P19), in contact with scaffolds containing bioactive glass nanoparticles, were more than scaffolds with no glass. The results of this study showed that CS/PVA/CNT/BG electro spinning nanocomposite scaffolds can provide appropriate mechanical properties and suitable fields for the cells growth and they are potentially an appropriate option to be used in neural tissue engineering.
None.
None.

©2016 Doostmohammadi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.