Journal of
eISSN: 2377-4282


Research Article Volume 7 Issue 3
Department of Biotechnology, Sapthagiri College of Engineering, India
Correspondence: Blessy Baby Mathew, Department of Biotechnology, Sapthagiri College of Engineering, 14/5 Chikkasandra, Hesaraghatta main road, Bengaluru-560057, Karnataka, India, Tel +919964716386, Fax 080–28 372800
Received: April 23, 2018 | Published: May 25, 2018
Citation: Mathew BB, Krishnamurthy NB . Evaluation of lead oxide nanoparticles synthesized by chemical and biological methods. J Nanomed Res. 2018;7(3):195 ? 198. DOI: 10.15406/jnmr.2018.07.00187
There is a pressing need for the development of standard and reference nanomaterials for environmental nanoscience and nanotoxicology. Green synthesis of nanoparticles with the use of microorganisms such as bacteria is a cost-effective and eco-friendly approach. In this work, the lead oxide nanoparticles (PbO–NPs) were synthesized chemically followed by the green synthesis using bacterial pellets of Bacillus toyonensis species. The formation of the nanoparticles was studied by using UV–vis spectroscopy method and the size of the nanoparticles was studied using particle size analyzer and X-ray diffraction technique using Debye-Scherrer equation. The average size of the nanoparticles during chemical synthesis resulted in a particle size of 1000nm whereas during biological synthesis the particle size was found to be in the size range of 200nm, 1000nm and above through the technique of Dynamic Light Scattering. Using Debye-Scherrer equation the particle size obtained through chemical synthesis was found to be 180nm whereas it was around 78 nm during biological synthesis.
Keywords: lead nanoparticles, lead toxicity, bacillus toyonensis, amalgamation, metal precursors
Development in the field of nanoparticle design and manufacture has implied that their characteristics can be conceivably modified to play out a particular errand and they are presently generally utilized as a part of industry and in business items. Their broad utilize implies that release to the earth is expanding.1 Nanoparticles (NPs) are characterized as constituents where no less than one measurement is in the vicinity of 1 and 100nm and are of incredible enthusiasm to a wide range of sciences and enterprises because of their novel physiochemical properties.2 In spite of the fact that the conduct and effects of these particles at naturally applicable settings has been to a great extent neglected.3 What's more, there is lack of controlled and appropriate tests for eco toxicological and natural examinations. Even reference materials are hard to find. Generation of such materials and learning of their conduct under pertinent conditions is an essential logical progress and will support a protected and maintainable nanotechnology industry.4,5 Synthetic strategies need utilization of lethal compound solvents, for example, lessening operators and in reality these techniques are costly. As of late in nanotechnology, researchers wish for green amalgamation with plant separates, since this strategy has been valuable and assumes a vital part which is substandard, spotless, safe, eco-accommodating, non-poisonous and with safe substances. Nanoparticles have numerous precise applications and new properties in various fields of medication; tranquilize conveyance frameworks, DNA marking, sensors, catalysis etc. In the green combination of nanoparticles, the phyto constituents are the obligation to decrease the span of nanoparticles such as proteins, phenols, starches, flavonoids, alkaloids and amino acids.6 By and large, the synthetic union procedure of nanoparticles ordinarily utilizes the accompanying three principle segments of metal precursors, reducing agents and soothing agents. The organization of colloids from the depletion of sample salts includes two phases of nucleation and subsequent growth. It is likewise uncovered that the size and the state of incorporated nanoparticles are explicitly subject to these stages. But during biological amalgamation of nanoparticles, the plummeting agent and additive are switched by particles formed by active organisms. Plants and microbes such as bacteria, fungi, yeasts and algae can be the source for these reducing or stabilizing compounds.7 The natural strategy gives an extensive variety of assets for the combination of nanoparticles, and this technique can be considered as an ecologically benevolent approach and furthermore as a minimal effort procedure. The rate of lessening of metal particles utilizing organic operators is observed to be significantly quicker and furthermore at encompassing temperature and weight conditions,8 during natural incorporation. The cell mass of the microorganisms pay a noteworthy part in the intracellular synthesis of nanoparticles. The adversely charged cell divider associates electrostatically with the distinctly charged metal particles and bioreduces the metal particles to nanoparticles,9 this resistance component can be exploited as a technique for nanoparticles synthesis and has points of interest over customary synthetic or conventional chemical routes. Nevertheless, it is not easy to have a huge quantity of nanoparticles using biological synthesis.
Lead oxide (PbO) nanoparticles have special assets and extensive variety of utilizations, for example, luminescent materials, gas sensors, stockpiling gadgets, modifiers and harmful ray protectant in oxide glasses. Among the different techniques accessible for the amalgam of lead oxide nanoparticles, the chemical procedure is exceptionally simple and helpful for fitting the dimension and morphology of the products. The surface area of the nanoparticles, rely on the interrelationship of molecule morphology and dimension, is a vital distinctive property for the nanoparticles including its capacity for ocular, electric, attractive, reactant exercises and so on. The tiny dimension of nanoparticles is an aspect supporting the agglomeration which expands the measure of nanoparticles. The bulkiness of nanoparticles alters their dimension, shape and modify the mixture in its elemental composition, physical characteristics and biological properties.10 A case of a bacterium that is remarkable for its capacity to lessen metal particles and live in conditions with or without oxygen is the metal-reducing bacteria that have an in-built capacity to join metal diminishment through the trait of digestion. Scanning electron microscopy and transmission electron microscopy pictures have uncovered anomalous basic distensions looking like bacterial fibers that are believed to be associated with the metal reduction or sorption. This procedure of creating an outside fiber is totally missing from the conservative microbial inhalation and is the focal point of numerous research studies.11 Microbes such as bacteria having usual ability to lessen and absorb heavy metals make it a entrant for use in wastewater management. Shewanella has been used to diminish a considerable quantity of palladium and de-chlorinate more than 50% of polychlorinated biphenyls.12 The bacterium's protection and utilization of heavy metal particles is profoundly identified with its digestion pathway. Presumed multidrug efflux carriers, proteins involved in purification, superfluous cytoplasmic sigma variables and PAS field controllers seem to have advanced articulation movement in nearness of substantial metal. Most nanoparticles are mass created, uncapped, have vast size appropriations and large size distributions.13,14 They are expected to combine or liquefy when exposed to natural water systems releasing ions, NPs and small aggregates. But bulky aggregates will deposit at the bottom, settle and stay in the water column, to be exposed to the environment.15,16 In certain studies it was mentioned that several nanoparticles are being widely used in naive form for some applications but the fact is that there is a mounting necessity for reduced size, well-defined particles with very precise and detailed property needs. The source of such variances and toxicity mechanisms is uncertain.5 It has been speculated that the journey amid oxidation conditions could exemplify the vast inconsistency in the toxicological information, instigating oxidative stress due to formation of free radicals. The impact of the media on the dimensions, conglomeration and surface oxidation condition of the particles is hardly depicted in several studies. A present restriction to advancement of additional learning is the absence of well-organized nanoparticles. Numerous nanoparticles utilized as a part of these examinations are ineffectively constrained and inadequately described.17,18 Reference materials used in various studies should be pure, mono dispersed, have a uniform size and synthetic in nature, as it is challenging to discern the particles when present at lower concentrations. 5 The extraordinary, physical, compound, and organic properties of nanometer-sized materials have as of late pulled in a lot of enthusiasm and a great deal of interest in the research community. Size and shape subordinate conduct of physical and chemical properties of materials have driven researchers to outline and create diverse techniques for integrating the nanomaterials. Among the physical and chemical properties that purposely depend on the variation in shape and size is light assimilation, attractive, magnetic and electrical qualities. Nanomaterials have been utilized as a major part of utilizations in sensors, catalysis, gadgets; surface upgraded Raman spectroscopy and in indicative imaging.19 The synthesis of nanomaterials be carried out by physical, chemical and biological methods by using plant or fruit extracts or by using microbial biomass in eco-friendly gentle surroundings. This paper reports the synthesis of lead oxide particles that were monodispersed aggregates and were stable in nature. The suspensions were characterized using particle size analyzer that was based on Dynamic Light Scattering, UV-vis spectroscopy and X ray Diffraction techniques.5
Apparatus
Laminar air flow cabinet, Orbital shaking incubator (Remi), Double beam spectrophotometer (Systronics 2202), Zetasizer nano ZS 90 (Malvern Instruments Inc.) and X ray diffractometer (Ultima IV- Rigaku).
Chemical synthesis
All tests were performed under climatic conditions. A lead nitrate solution of 0.02M was prepared using polyvinyl pyrrolidone (10g/L) as a structure added substance and the solution was sonicated for 30min and followed by this the addition of sodium hydroxide was gradually done.20 The lead hydroxide was formation took place as indicated in the equation below:
Pb(NO3)2 (aq) + 2NaOH (aq) → Pb(OH)2 (s) + 2NaNO3 (aq) (1)
The blend was sonicated for 30min, followed by filtration of precipitated lead hydroxide that was washed with distilled water and ethanol for three times. The attained precipitate was hosted into ethanol followed by sonication for 30min and filtration. The last precipitate acquired after this was dehydrated at 320°C for 3h, and during this stage,20 the following reaction took place:
Pb(OH)2 (s) → PbO (s) + H2O (g) (2)
Biological synthesis
Bacterial strains of Bacillus toyonensis was grown aerobically using Luria-Bertani broth in separate 250ml Erlenmeyer flasks containing 25mL of broth at 37°C for 35h on a rotary shaker (125rpm). The bacterial pellets obtained after centrifugation at 10000rpm for 10 min were separated from the supernatant, washed with deionized water multiple times, dried and then used for biosorption experiments.21 Lead nitrate solution (0.02M) was prepared in two different 250ml Erlenmeyer flasks and bacterial pellet weighing 0.1gm was introduced into it. The absorption spectra and particle size before introducing the pellet was evaluated.
UV–vis spectroscopy
UV/Vis spectroscopy determines the concentration of the solute in a solution that works towards absorption. As the concentration varies, the absorbance also changes. This is based on the Beer-Lambert law that states the absorbance of a solution is directly proportionate to the amount of the absorbing species in the solution. The formation of nanoparticles was confirmed by UV-Visible spectroscopy using Systronics double beam 2202 spectrophotometer instrument, that have two lamps namely, halogen and deuterium to analyze the visible and ultra-violet spectra. The mode of measurement was selected as absorbance and the scan speed was set as medium for all the sample sets. The lead nanoparticles were analyzed with UV-Spectrometer in the range of 230 nm to 370nm. It was confirmed by the position of absorption peak that the lead nanoparticles were produced during the chemical as well as biological synthesis.22
Particle size determination
By judging the arbitrariness in the strength of the scattered light released by a suspension, the size and distribution of the particles in a suspension can be known. This can be accomplished by the technique of dynamic light scattering (DLS) that is based on Brownian motion. The particles present in the suspension will be in movement and continually in interaction with the solvent molecules and this bombardment affects the speed of the particle. The smaller the particle is, the quicker its Brownian motion would be resulting in higher speed. Particle size measurements were done to evaluate the lead particles generated by chemical synthesis and green synthesis methods.23
X ray Diffraction
The crystalline structure of the nanoparticles were determined by X-Ray diffraction analysis using Rigaku X-Ray diffractometer (Miniflex, UK) instrument operating at 40kV with 2sec time interval with wavelength of radiation (λ = 1.5406Å), at 27°C.24
Particle size determination
Particle size distribution by intensity is given in Figure 1. This suggests that the chemical synthesis resulted in a particle size of 1000 nm where around 28% of the particles were within that range (Figure 1A). Figure 1B suggests that the biological synthesis also resulted in a particle size of 1000 nm but only 17% of the particles were within that range. Around 11% of the particles were within the range of 200 nm and less than 2% of the particles were within the range of 10000 nm. Even though the mean diameter was high, the difference in the values may be due to the presence of aggregates. The reproducibility and yield of the nanoparticles was good during the biological synthesis process.
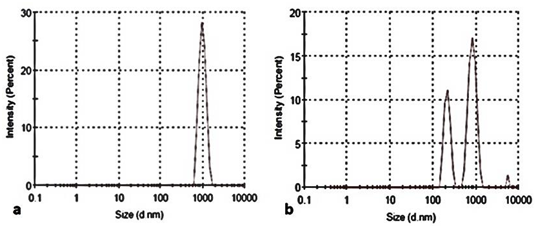
Figure 1 Particle size distribution of the nanoparticles produced by (a) Chemical synthesis (b) Biological synthesis.
UV–vis spectroscopy
It was observed the particle size was small at low concentration of lead, whereas the particle size was found to increase due to agglomeration when the lead concentration was escalating. There were several mechanisms that were actively taking part in this transition. 0.02M solution of lead nitrate was kept as optimum concentration as below it there was not much change recorded in particle size and morphology. The concentration of sodium hydroxide was 0.01M to 0.4M. The result obtained from UV-Visible spectroscopy analysis of the sample is presented in Figure 2. It is the most important method for detecting the Surface Plasmon Resonance property of nanoparticles. During the chemical synthesis the maximum absorption was recorded from the peak at 210nm (Figure 2A), whereas during biological synthesis the maximum absorption was seen from the peak at 220nm (Figure 2B).
X ray diffraction
During chemical synthesis, diffraction peaks were obtained at 2θ values of 31,44,52 and 55 using XRD spectrum analysis (Figure 3A). On the other hand, during biological synthesis, diffraction peaks were obtained at 2θ values of 26,30,43 and 51 using XRD spectrum analysis (Figure 3B). To determine the size of the nanoparticles, the Debye-Scherrer equation25 is used which is given below:
where D is the diameter of the particle, K is Scherrer constant, λ is X-ray wavelength (0.1541nm), β is the complete width at partial extreme of the diffraction peak and θ is the Bragg’s angle. The average crystallite sizes of the PbO particles were calculated according to Debye-Scherrer equation and the average particle size was found to be 180NM during chemical synthesis whereas it was around 78NM during biological synthesis.
Lead nanoparticles were successfully synthesized by using chemical and biological methods of synthesis, which provides cost effective, easy and proficient way for synthesis of nanoparticles. The particles produced via biological synthesis were found to be tinier, abundant and of varied size when compared to the chemically synthesized particles. This made them suitable to be used in biomedical, wound healing, drug delivery and drug-embedding applications. The biological method of synthesis is suitable to produce well-characterized, eco-friendly and stable nanoparticles that do not need any external stabilizers or toxic reactants. On the other hand, the chemically synthesized lead nanoparticles could be used for plenty of applications such as in making electrodes, lead batteries etc. Use of the NPs in toxicology tests will therefore reflect the particle behavior and not the dissolved metal behavior. Taken together the lack of aggregation and dissolution suggest that these materials will make excellent model compounds for studies in ecotoxicology and surface water environmental engineering.
The authors wish to acknowledge the lab facilities provided by the Department of Biotechnology, Sapthagiri College of Engineering (SCE), Bangalore to carry out the research work and we also thank the Principal and Management of SCE for their constant encouragement and support.
Authors declare there is no conflict of interest.

©2018 Mathew. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.