Journal of
eISSN: 2377-4282


Research Article Volume 5 Issue 4
Division of Natural and Mathematical Sciences, LeMoyne-Owen College, USA
Correspondence: YZ Hamada, Division of Natural and Mathematical Sciences, LeMoyne-Owen College, 807 Walker Avenue, Memphis, TN 38126, USA, Tel 1(901) 435-1392, Fax 1(901) 435-1424
Received: March 30, 2017 | Published: May 9, 2017
Citation: Hamada YZ, Makoni N, Hamada H (2017) Cu 2+ Complexes with the Simplest Amino Acid Glycine (Gly). J Nanomed Res 5(4): 00123. DOI: 10.15406/jnmr.2017.05.00123
Using potentiometric titrations, UV-Vis, IR and speciation diagrams, it appeared that the simplest amino acid Glycine (Gly) is not reacting in a simple manner at all with the copper metal ion (Cu2+) in aqueous solutions at 25˚C. The potentiometric measurements indicated that Cu2+ released a net of two protons (2H+’s) into the solution. Free Gly released one proton (H+) into the solution from the single ammonium group. On the other hand, when Glycine hydrochloride (Gly.HCl) was used instead of free Gly, both the carboxylate and the ammonium groups released their protons. Upon the reaction of Cu2+ with Gly.HCl in any molar ratio, a net of four protons (4H+’s) or more were released into the solution; one H+ from the carboxylic acid group, the second from the ammonium group and the additional two H+’s from the Cu2+-aqua ligands. The proposed solution species are in a good agreement with what has been shown in the literature.
Keywords: Aqueous solutions, Glycine, Potentiometry, Zwitterion
Most Biology/Chemistry/Physicists and Medicinally related researchers think that the commonly known 20 Amino acids (AA) have been studied to the extent that they know almost everything about them. We believe that studying the simplest amino acid Glycine (Gly) is not that simple when it comes to its reactions with metal ions especially in aqueous solutions under ambient conditions. It is known that Gly is an inhibitory neurotransmitter.1-3 Typically a 70 kg human body contains about 280 mg copper (Cu2+). The copper ion concentration in seawater is in the range of one micro-molar or (1.0x10-3 mM), while the human Extracellular Blood Plasma concentration of Cu2+ is ~1.5 x10-2 mM.1
A very recent chapter by Farkas and Sovago indicated the appearance of 400 papers that discussed metal-complex formation of simple AA and short peptides during the two years span of 2014 & 2015.4 The Gly/Cu2+ interaction was studied in the following reports5-9 at which the simple one-to-one [Cu2+-Gly] and the bis- [Cu2+(Gly)2] complexes were identified unanimously. Herein, we are showing a very detailed potentiometric and a semi-detailed spectroscopic study (UV-Vis and IR-spectroscopies) that confirm the presence of these complexes observed previously.4-9 We have seen the appearance of the new ternary [Cu2+-Gly-(OH)2] complexes. We have recently published two reports that discussed the interactions of copper with an important Phenoxy-mono-carboxylate (clofibric acid) and the most famous mono-hydroxy tri-carboxylate (citric acid).10,11 In these reports we have shown that there is huge body of literature that dealt with the chemistry of copper as one might expect (Figure 1).
Chemicals/solutions
All solutions were prepared using 99% purity Sigma reagent grade free Gly formula weight (FW) 75.1 g.mol-1 or Glycine hydrochloride Gly.HCl FW 111.5 g.mole-1. Cu2+ solutions were prepared using Copper sulfate penta-hydrate, Cu(SO4).5H2O, formula weight 249.68 g.mol-1. All solutions were prepared by using doubly deionized water. The pH values of all solutions were adjusted using (0.09064 ± 0.00104 mol.L-1) sodium hydroxide (NaOH) solution. The pH values were measured using Orion Membrane pH meter (model 720) with a combination Orion-glass electrode in 0.0 mole.L-1 ionic strength (I).
Preparation of the potentiometric titration solutions
In all free Cu2+, or free Gly, or free Gly.HCl, or Cu2+ -Gly, or Cu2+-Gly.HCl potentiometric titrations in 1:1, and 1:2, and 1:3, and 1:4, and 1:5 ratios, NaOH solution was the titrant. NaOH solutions were prepared from NaOH laboratory grade pellets in carbonate free water. The methods used to prevent the contamination of the titrant with atmospheric CO2 had been described elsewhere.10-15 The NaOH solutions were standardized using primary standard potassium hydrogen phthalate (KHP). Both NaOH and KHP were purchased from Fisher Chemical Co. Before any KHP titration, the KHP was dried at 110˚C for 24 hours and stored in a desiccator. A stock indicator solution of about 0.2% phenolphthalein in about 90% ethanol was prepared from reagent grade phenolphthalein. KHP was titrated to the phenolphthalein end point. Typically, thirteen-fifteen runs were carried out to standardize the NaOH solution. Standard statistical treatments of the data such as the arithmetic mean, standard deviation, T-test, and Q-test were conducted using Excel software.
Potentiometric titrations
The potentiometric titration solutions were contained in a 250 mL beaker equipped with a magnetic stirring bar. The beaker was covered with a custom made Teflon cover. In a typical titration; the Gly or Gly.HCl solutions were added first (in independent experiments) followed by the addition of Cu2+ solution. The mixture was allowed to stand for a minimum of five minutes to reach a state of equilibrium. No other solutions were added to adjust the ionic strength of the solution. The total volume of the final titration solution was 100 mL. The final concentration of the Cu2+ ion titrated was in the range of 2.0 mmoles.L-1. Before each titration, the titration solution mixtures were allowed to stir for an extra 25 minutes for complete equilibrium.
The NaOH titrant was added in segments of 100 mL increments using an Eppendorf micropipette with continuous stirring. The time intervals between the additions of the NaOH solution were set to 5 minutes, which was sufficient to get each of the pH values stabilized and reach complete equilibrium. The start pH-value was in the range of 3-4 (unless otherwise is specified) and the final pH-value was in the range of 10-11. Each titration took about 5 to 6 hours to complete. All titrations were conducted at room temperature.
UV-Vis spectroscopy
We have gathered all UV-Vis spectroscopy measurements on the T60 high-performance spectrophotometer in connection with UVWIN software version 5.0, both purchased from Advanced ChemTech (Louisville, KY). UV-Vis Samples were prepared in D.I. water at 25˚C. The entire UV-Vis spectrum was scanned from 250 to 1000 nm using quartz cuvettes with optical path length of 1 cm. A reference cuvette filled with D.I. water was used with all measurements. The concentration of Cu2+ was = 8.75×10−3 mol.L−1. The UV-Vis spectra were collected at the pH values of 3.00 after 60 minutes equilibrium time and the measurements were repeated after 1440 minutes (24 hours equilibrium time) to ensure complete equilibrium.
IR spectroscopy
All IR spectroscopy measurements were conducted using Nicolet iS10 spectrophotometer in connection with OMNIC software version 8.1, both purchased from Thermo Fisher Scientific (Madison, WI). Samples were prepared in D.I. water at 25˚C. The entire IR spectrum was scanned from 400 to 4000 cm-1 using the provided attenuated total reflectance (ATR) accessory cell compartment equipped with a diamond cell that can accommodate solid samples or aqueous solution samples. The following data parameters were used in collecting the IR spectra: number of sample scans and the number of background scans was set at 32 with resolution of 4.000, and Laser frequency of 15798.7 cm-1. Typical IR spectra were generated in which the X-axis was given as Wave numbers in cm-1 and Y- axis was recorded as % Transmittance.
Potentiometric titrations of free Gly, Gly.HCl, and free Cu2+ ion
Figure 2 is the potentiometric titration experiments of free Gly.HCl, which shows plots of three independent titrations at which the acidity constants of both the carboxylic acid functional group and the ammonium groups are separated by a well-defined sharp inflection point. Figure 3 is the speciation diagram of free Gly.HCl generated in aqueous solutions using Hyperquad simulation and speciation (Hyss) software program,16 pKa values were used from Martell & Smith,17 pKw value of 13.78 was taken from the literature.18 Gly.HCl releases a net of two protons due to the fact that Gly.HCl has two titratable functional groups; the carboxylic acid (-COOH) group and the ammonium (NH3+) group as shown in Figure 2. Data of this ligand has been reported in the NIST standard reference database of critically selected stability constants of metal complexes.17 Data about the reaction of Cu2+ and Gly.HCl are catalogued in Table 1.
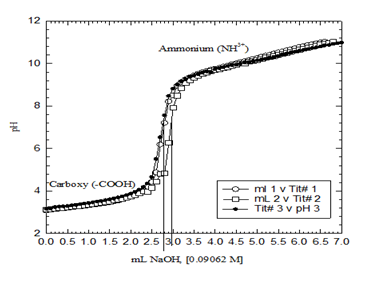
Figure 2 Potentiometric titration graph of free Gly.HCl (F.wt = 111.5 g/mole). Three overlapped plots are shown to prove data consistency. The carboxylate proton was intact before the addition of the first point (100 μL) of the titrant (NaOH) in that case.
|
Cu2+/Gly Compounds |
Log β ± SDa |
Log β ± SDb |
Net Charge |
Remarks |
|
H2Gly |
2.32 ± 0.01 |
2.33 ± 0.01 |
1 |
c |
|
HGly |
9.62 ± 0.01 |
9.57 ± 0.01 |
0 |
c |
|
Cu-Gly |
8.28 ± 0.01 |
8.19 ± 0.04 |
1 |
c |
|
Cu-(Gly)2 |
15.38 ± 0.01 |
15.1 ± 0.01 |
0 |
|
|
Cu-(Gly)3 |
19.55 ± 0.03 |
- |
-1 |
|
|
Cu-(Gly)H-1 |
1.28 ± 0.01 |
- |
0 |
|
|
Cu-(Gly)H-2 |
-9.25 ± 0.02 |
- |
-1 |
c |
|
Cu-(Gly)2H-1 |
4.91 ± 0.05 |
- |
-1 |
|
|
Cu-(Gly)2 H-2 |
-4.96 ± 0.04 |
- |
-2 |
Figure 4 is the potentiometric titration graph of free Gly. Three titration plots were overlapped to show data consistency. The initial pH of the solution was about 8.50 which are totally different compared to that shown in Figure 2. This is due to the fact that free Gly shown in Figure 4 has lost its carboxylic acid proton before the addition of the first increment of NaOH titrant. In another word, free Gly exists in its Zwitterion form. So that the degree of protonation or de-protonation of the reacting ligand is a governing factor for the identity of the metal complexes, or nano-metal species, or medicinal, or chemical species formed.
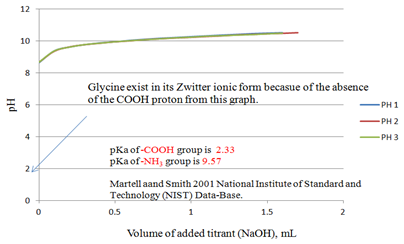
Figure 4 Potentiometric titration graph of free Gly (F.wt = 75.1 g/mole). Three overlapped plots are shown to prove data consistency. The carboxylate proton was already dissociated before the addition of the first point (100 μL) of the titrant (NaOH) in that case. pKa-values shown are from Martell and Smith published by NIST.17
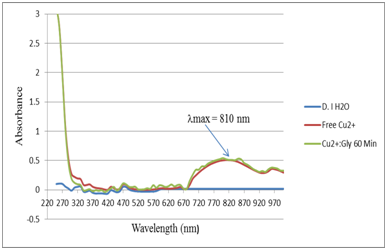
Figure 5 UV-Vis absorption spectra for the control (DI H2O), Free copper sulfate (Cu2+) and Cu2+:Gly in 1:1 ratio after 60 minutes equilibrium time
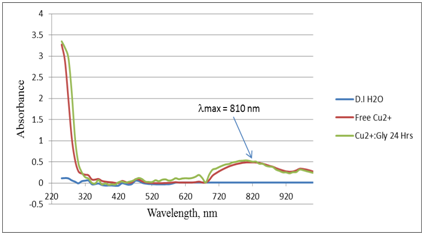
Figure 6 UV-Vis absorption spectra for the control (DI H2O), Free copper sulfate (Cu2+) and Cu2+:Gly in 1:1 ratio after (24 hours) or 1,440 minutes equilibrium time.
We have shown in the supplementary material the detailed potentiometric titrations of free phosphoric acid (H3PO4) and that of free Cu2+ solutions (Supplementary Figures 1-6) in which the total number of protons released by each species is shown. For example, titrating free Cu2+ releases a net of two protons (2H+) or two equivalents into the aqueous solutions. This is due to metal ion hydrolysis. This term is defined in equations 1-218-20 and it is valid for any metal ion in aqueous solutions. The number of equivalents is defined as the number of milli-moles of added titrant (NaOH in this case) per number of milli-moles of metal ion present in solution (Cu2+ ion in this case).
[Cu(H2O)6 ]2+→ [Cu(H2O)5(OH)]++ H+ (1)
[Cu(H2O)5OH]+→ [Cu(OH)2]ppt + H+ (2)
Potentiometric titrations of Cu2+ with Gly.HCl in various molar ratios (1:1, 1:2, 1:3, 1:4, and 1:5 ratios)
Supplementary Figures 7-14 are the detailed potentiometric titration graphs of the Cu2+:Gly.HCl in 1:1, 1:2, 1:3, 1:4, and 1:5 molar ratio respectively. These graphs contain a total of ten individual plots. This graph shows the exact locations of the inflection points. The location of each inflection point gives the exact number of protons released into the aqueous solution. For example, the titration plots of the Cu2+: Gly. HCl in 1:1 molar ratio indicated the release of four protons. By examining these plots in this figure compared to that for the free Cu2+ graph, clearly there has been a strong interaction between the metal ion Cu2+ and Gly.HCl solutions due to the shift in the location of the inflection points to 4.0 equivalents compared to 2.0 equivalents as shown in the titration of the free Cu2+ ion in Figure 7 of supplementary material.
Every potentiometric titration graph for each molar ratio is followed by another Figure that shows the mathematical treatment plots for each potentiometric graph. For example, Supplementary Figure 7 is followed by Supplementary Figure 8 which is the mathematical treatment or the first derivative (slopes pH/V) versus the number of observed equivalents.
It will suffice to discuss the 1:1 titrations (Cu2+: Gly.HCl) as an example, in which the three replicas overlapped at 4.00 equivalents. The important point here is that four equivalents of protons have been released from the reaction of Cu2+ with Gly.HCl and went into the solution. Two protons were clearly released from the Gly.HCl. The source of the other two protons must be accounted for. These two protons came from the aqua ligand attached to the Cu2+ ion. It is established in the literature that such hydroxo-complexes with Cu2+ have been observed previously.8,10,17-20 The proposed and the most plausible species to be formed in solution will be the ternary copper hydroxo-glycinate complex [Cu2+(Glycinate-)(OH-)2]1-. Any complex we have observed in the current study is shown in Table 1 to be compared to the literature values. Table 2 is the summary of all potentiometric titrations carried out in the current study.
|
Cu2+:Gly ratios |
Volume of Added Titrant (mL) |
No. of Equivalents of Titrant (Eq.) |
Remarks |
|
0:01 |
1.65 ± 0.07 |
1.05 ± 0.07 |
1 Proton (H+) released |
|
1:00 |
2.17 ± 0.06 |
1.97 ± 0.06 |
2 Protons (H+) released |
|
1:01 |
4.70 ± 0.17 |
4.18 ± 0.16 |
4 Protons (H+) released |
|
1:02 |
6.40 ± 0.14 |
6.15 ± 0.20 |
6 Protons (H+) released |
|
1:03 |
7.70 ± 0.01 |
7.38 ± 0.06 |
7.4 Protons (H+) released |
|
1:04 |
9.03 ± 0.29 |
8.74 ± 0.27 |
8.75 Protons (H+) released |
|
1:05 |
10.95 ± 0.07 |
10.55 ± 0.13 |
10.55 Protons (H+) released |
Table 2 Summary of all potentiometric titrations of Cu2+: Gly.HCl in 0:1, 1:0, 1:1, 1:2, 1:3, 1:4, and 1:5 moalr ratios (ionic stregnth I = 0.0).
High equilibrium UV-Vis spectroscopy of Cu2+ with free Gly
We have conducted novel UV-Vis absorption spectroscopy experiments. In these experiments, Cu2+ was reacted with the free Gly that was potentiometrically titrated in Figure 4. The Cu2+ solution was mixed with Gly solution in 1:1 molar ratio. Figure 5 shows the UV-Vis absorption spectra for the control (DI H2O), free copper sulfate solution (Cu2+) and Cu2+:Gly solution in 1:1 ratio after 60 minutes equilibrium time. The experiment was repeated after 24 hours on the same set of cuvettes to observe if there were any changes in the absorption pattern of the Cu2+:Gly reaction system after a very long equilibrium time i.e. 1440 minutes. Figure 6 shows the UV-Vis absorption spectra for the control (DI H2O), free Cu2+ solution and Cu2+:Gly solution in 1:1 ratio after (24 hours) or 1,440 minutes equilibrium time. It is noteworthy that researchers in6,7 showed some UV-Vis absorption spectra for copper Glycine systems however none were similar to the spectra presented in the current study.
The absorption peaks that shown had a maximum absorption peak at λmax = 810 nm, (Absorbance value of 0.521) which is the typical region for the d9 metal ion such as Cu2+.19 With a simple Beer’s-Lambert equation calculation one can calculate the molar absorptivity (ε) as shown in equation (3).
A = ε c l (3)
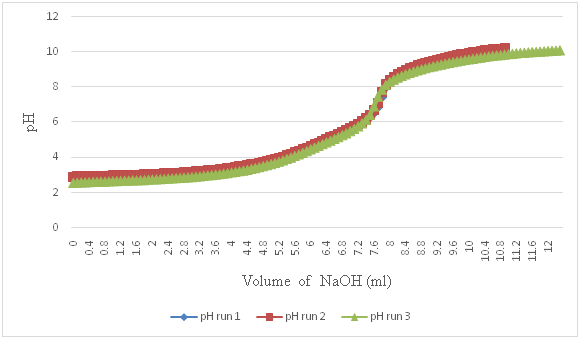
Figure 7 Potentiometric titration graph of Cu2+: Gly.HCl in 1:3 molar ratios. Three plots were overlapped to prove data consistency. Summary of all Cu2+:Gly in 0:1, 1:0, 1:1, 1:2, 1:3, 1:4, and 1:5 molar ratios is given in Table 2.
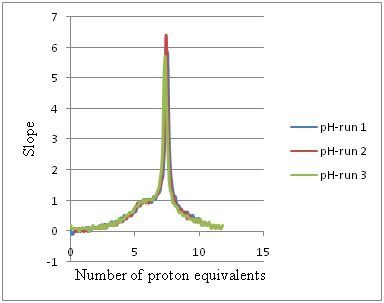
Figure 8 First derivatives of the potentiometric titration graphs showed in Figure 7 to measure the number of proton equivalents released into solutions due to the interaction of Cu2+ with Gly, HCl in 1:3 ratios. Table 2 shows the summary of all Cu2+:Gly in 0:1, 1:0, 1:1, 1:2, 1:3, 1:4, and 1:5 molar ratios.
Where (A) is the measured absorbance, (ε) is the molar absorptivity in M-1cm-1, (c) is the molar concentration and (l) is the optical path length (1.0 cm) in that case. It turned out that (ε) λ810nm = 14 M-1cm-1 because (c) of [Cu2+] = 0.0350 M. also using the equilibrium constant for the 1:1 complex, Log Keq.1:1 = 8.24 averaged from references8,17 (Table 1) one can calculate the free energy change ΔG according to equation (4).
ΔG = -RT LnKeq.1:1 (4)
At room temperature, T = 25˚C (298K) and R = 8.314 J.mole-1K-1 and Keq.1:1 = 108.24 the value (K eq. of 8.24 is the averaged vales from the two values in Table 1, 8.28 and 8.19) accordingly, ΔG = - 4.3 x 1011 J.mol-1 which is indicative of spontaneous and favored reaction.16-20
IR Spectra of free Gly with Cu2+
Supplementary Figure 15 shows the overlaid IR-Spectra collected for air (showing the characteristic peaks for CO2 at 2,360 cm-1) which was absent from the rest of the samples. The main peak that changed due to the binding of Cu2+ to Gly is the carbonyl peak of the carboxylate functional group that appeared at 1,577 cm-1. There were no dramatic changes in the locations of the peaks of the free Gly to that of the Cu2+-Gly mixture however the intensities of all observed peaks for free Gly were diminished due to the reaction of Gly with the copper metal ion.
In the current report, we augmenting the current literature data presented, thus far, on the reaction of the copper metal ion with the simplest AA, Gly. We confirm the appearance of the ternary copper glycinate di-hydroxo complex in aqueous solutions. We are very confident that the following reaction (Equation 5) is valid representation of what took place when Cu2+ reacted with Gly.HCl.
[Cu(H2O)6 ]2++ Gly.HCl → [Cu(H2O)4(Gly)(OH)2] + + 4H+ (5)
We have reached the conclusion given in equation (5) due to the fact that in the reaction of Cu2+ with Gly.HCl in 1:1 ratio, a net of four protons were observed at the inflection point. The shift of the inflection point from 4 to 6 in the next set of titrations i.e. the 1:2 reaction of Cu2+ to Gly.HCl is due to the presence of an extra mole of glycine hydrochloride. As the number of moles of Gly.HCl is increased the number of proton equivalents increased accordingly.
The fact that the UV-Vis absorption spectra of the reaction mixture of Cu2+ with Gly.HCl in 1:1 ratio did not change its absorption peaks at 810 nm even after 1440 minutes of equilibrium time is an indication of the robustness of the formed copper glycinate complex (es). Data from reference 8 showed that more than a half dozen copper glycine complexes formed. In their study, they used a calculation program as well as an experimental approach. In our recent study of Cu2+ with clofibric acid, we have shown a detailed IR spectra of all peaks that appeared and changed its stretching frequencies due to binding to Cu2+.10 Due to the fact that there was dramatic decrease in the intensity of the stretching frequency of the carbonyl C=O group of the carboxylate at 1,577 cm-1, we concluded that there was binding via this carbonyl which was supported by the calculations in.9
This work was supported in part from NSF under Grant # HRD-1332459. Special acknowledgements go to the financial support of the ACS-SEED summer program to Hasan Hamada.
None.

©2017 Hamada, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.