Journal of
eISSN: 2377-4282


Mini Review Volume 6 Issue 3
Chemical and Biological Engineering Department, University of New Mexico, USA
Correspondence: Sofia Babanova, Chemical and Biological Engineering Department, University of New Mexico, USA, Tel 505 358 8044
Received: September 01, 2017 | Published: October 3, 2017
Citation: Babanova S (2017) Bioelectrocatalysis and More. J Nanomed Res 6(3): 00155. DOI: 10.15406/jnmr.2017.06.00155
Bioelectrocatalysis is a phenomenon widely explored by research community in various directions: biochemical assays for quantitative determination of different compounds of interest, food and pharmaceutical industry, cosmetics, production of detergents, wastewater treatment, etc. All these applications rely on the advantages of enzymatic and bacterial catalysis: specificity, selectivity, fast reaction rate and regulation capacity. The same advantages have been explored in the design of bioelectrochemical systems for energy conversion, biosensing applications and wastewater purification.
Bioelectrochemical systems also known, as biofuel cells are one of the most popular “non-conventional” fuel cells in the last 50 years and the most promising technology for conversion of renewable biomass to electrical power as well as wastewater treatment approach.1,2 Biofuel cells can be divided into two main categories: enzymatic fuel cells (EFC) and microbial fuel cells (MFC). In both, oxidation of a given “fuel” occurs at the anode combined with a reduction of final electron acceptor at the cathode.3 The potential difference between the two electrodes is the driving force of the processes, which leads to the transformation of chemical energy, stored in the “fuel” bonds, to electrical current.4 Based on their operational principle these systems are classified as galvanic or fuel cells but what makes them “untraditional” is the nature of the catalysts used. In EFCs, the oxidation and reduction processes are catalyzed by the utilization of specific redox enzymes and in MFCs, the catalysts applied are microorganisms. The exploration of naturally occurring processes and phenomenon for the generation of electricity is the most beneficial feature of biofuel cells.5 They are biocompatible, cheap, selective, and effective at mild temperatures and neutral pH. Therefore, biofuel cells can be a key technology toward the generation of clean and sustainable energy.
Many research efforts have been dedicated to the development and improvement of bioelectrochemical systems, specifically fundamental understanding of bioelectrocatalysis and internal and external electron transfer, materials selection, and design optimization. Based on the gained knowledge through the years, the generated power and current densities from laboratory prototypes of biofuel cells have increased significantly (from 0.05 mA/m2 to 1000 mA/m2).6 However, after 100 years of research in this field, scientists and engineers have not yet realized dramatic improvements in energy densities and/or huge achievements as practical devices. There are two main reasons for that:
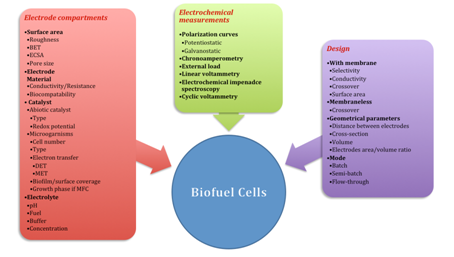
Figure 1 Factors determining bioelectrochemical systems performance.7
Bottom up approach: Integration of biocatalysts with different solid supports for enhanced interfacial interactions
The bottom up approach for bioelectrochemical systems starts with fundamental study and optimization of biocatalyst-support interactions as the main rate limiting step for enhanced electron transfer rate and improved performance. These include but are not limited to the development of various techniques for sufficient enzyme immobilization trough physical adsorption.8 covalent attachment.9 physical entrapment and tethering of enzymes.10 Mediators have been used to enhance electron transfer rate.6,9,11 and carbon or mesoporous materials with diameters comparable to the size of the enzyme were explored for enhanced enzyme–electrode interactions.12 The following approaches provide proper enzyme orientation: specific covalent attachment of enzymes to the electrode surface.13,14 protein engineering.15 and reconstitution of apo-enzymes directly on the electrode surface.16
A new approach for improved enzyme-electrode interactions and ultimately for enhanced bacteria-electrode electron transfer has been developed.17-19 This approach is based on the specific enzyme-substrate interactions, relying on key-lock principle that is the main advantage of enzymatic catalysis. Depending on the enzyme type two approaches were developed. The first approach uses modification of the electrode surface with enzyme`s natural substrate or its functional analogue, which ultimately results in proper enzyme orientation and facilitated electron transfer (Figure 2). This hypothesis was applied for the orientation of two of the most famous oxygen-reducing enzymes, Bilirubin Oxidase and Laccase and its rationality was demonstrated in the development of oxygen reducing electrodes.17,18 Further this hypothesis was transferred towards enhancement of electron transfer from “anodic” enzymes towards solid supports, where the enzyme terminal electron acceptor was utilized for electrode surface modification. This approach led to eight fold increase in the performance of an anode composed of pyrroloquinoline quinone (PQQ) dependent glucose dehydrogenase, very promising enzyme for the design of glucose sensors.20,21

Figure 2 MCOs orientation based on the key-lock principle.18
Currently research activities associated with the utilization of enzymes in bioelectrochemical systems are focused on understanding of the coenzyme location and structure as well as its participation in internal and external electron transfer. What is not known is: How these enzymes interact with their substrates or final electron acceptors in nature?; What structural changes accompany the enzyme immobilization and operation in biofuel cells?; What is the nature of enzyme-substrate recognition mechanism and interactions?; How this can be explored in the development of enzymatic electrodes with enhanced enzyme-electrode interactions?; Is the final electron acceptor diffusing into the enzyme molecule?; Is there a specific acceptor-binding pocket?; Is the same channel used for substrate and electron acceptor transportation, or there is another path for the acceptor to get close to the reduced cofactor?; What is the mechanism of the cofactor-electron acceptor interactions?; What happens when the enzyme contains more than one cofactor?; etc. These are some of the fundamental questions we need to find answers to and explore the knowledge in the development of practical systems based on enzymatic catalysis. The knowledge gained can be further transferred in the development of microbial electrodes and improvement of the bacteria-electrode communication.22
Multi-parameter approach: Identify and isolate the factors having the biggest impact on the operational features of bioelectrochemical systems
Once the biological interfacial reactions are enhanced and are no longer the rate-limiting step in the operation of biofuel cells, understanding of the design parameters and their influence becomes a primary question.23 Therefore, it is highly recommended to identify the key factors having the biggest impact on system operational features as well as system uncertainty. Unfortunately, due to the complexity of biofuel cells and mainly because of their interdisciplinary character, researchers consider the most important factors to be in the range of their knowledge and interests. Microbiologist and biochemists consider the biological component as determining BFCs operation. At the same time, engineers consider the design parameters as the most important once. Who is right?
To find the answer of this question slightly different approach needs to be explored. This approach is highly innovative in this field and it relies on statistical analysis and data interpretation. Through evaluation of the expanded uncertainty of microbial fuel cells, the variations in electrodes resistance and mostly difference in their electrochemical accessible surface area were identified as the main parameters causing irreproducibility of MFCs responses.24,25 The idea of using statistical approaches for analysis of biofuel cells was further enlarged to include Design of Experiments, Principal Component Analysis (PCA) and Canonical Correspondence Analysis.7,26-29 The applied statistical interpretation of the results from a significant amount of MFCs data showed that design parameters were the dominating factors when the necessity bacterial biofilm was developed. The same conclusion was made when the expanded uncertainties of the main operational characteristics were evaluated (Figure 3).28

Figure 3 Uncertainties of the MFCs operational characteristics and several MFCs parameters. The uncertainty values in the pies are not normalized to 100%.28
Biofuel cell design parameters along with the operational conditions can be varied to emphasize the influence of a factor over the systems performance, not just in terms of electrochemical output but also towards other specific applications such as wastewater purification, quantitative determination of target compounds, etc. Identifying the main factors determining the biofuel cell performance, Principal Regression Analysis in combination with Path analysis can be used to build a mathematical model that can predict the final response of a system with a given parameters. This will dramatically decrease the experimental time and efforts for their optimization. Of course, to transfer a research idea into a technology, one main requirement should be fulfilled – reproducibility. Without reproducible performance, practical application cannot be possible.
Expanded uncertainty is a new term in the field of biofuel cells, which was recognized as necessary and introduced for the interpretation of results first from microbial fuel cells 24,25. As for conventional fuel cells, the commonly used electrochemical techniques for MFCs operational characteristics determination are polarization and power curves. The results from these polarization measurements are used for decision making if the studied fuel cell is appropriate for the intended purpose or not. Thus, uncertainty estimation of operational characteristics is required by the contemporary regulations for proper result presentation and comparison between results obtained in different laboratories under different experimental conditions. Study of the contributions of various uncertainty sources to the combined uncertainty allows to perform optimization of the experimental parameters having highest contribution to the uncertainty and, thus to obtain more reliable electrochemical system with reproducible output values 25. This is a question of crucial importance for the future practical application of biofuel cells especially when biosensors are developed.
The design and optimization of bio-electrodes and biofuel cells is not trivial. This technology bridges variety of research areas such as biotechnology, energy harvesting and generation, environmental science, micro- and nanostructured materials. Therefore, innovative and untraditional approaches need to be considered and combined.
Most the achievements referenced in this paper are a result of the work of two very important scientists, Prof. Plamen Atanassov and Dr. Orianna Bretscgher, to whom I am much obligated.
The authors declare no conflict of interest.

©2017 Babanova. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.