Journal of
eISSN: 2377-4282


Research Article Volume 5 Issue 2
1Universidad del Ejercitoy Fuerza Aerea, Mexico
2Instituto Politecnico Nacional-Escuela Superior de Medicina, CDMX, Mexico
3Instituto Nacional de Medicina Genomica, Mexico
4Departamento de Medicina Experimental, Facultad de Medicina, Mexico
Correspondence: Cesar A Gonzalez Diaz, Instituto Politecnico Nacional-Escuela Superior de Medicina, Ciudad de Mexico, Mexico,, Tel (52) 55 5729-6000 Ext. 62794
Received: January 23, 2017 | Published: February 22, 2017
Citation: Alvarez CPC, López GCV, Jurado VQ, Zayas MEG, Díaz CAG (2017) Assessment of GRB2 Dynamics vs. Bioimpedance Measurements as a Function of Systemic Infusion of “Magnetic Nanoparticle - Anti-HER2” in an Experimental Breast Cancer Model. J Nanomed Res 5(2): 00107. DOI: 10.15406/jnmr.2017.05.00107
Breast cancer (BC) is the most common malignancy among the female population. Ablation Radiofrequency (RF) assisted with magnetic nanoparticles (MNPs) coupled to a monoclonal antibody anti-HER2 (Human epidermal growth factor receptor 2) has been proposed as an alternative therapeutic technique, the concept behind the technical proposal is to change the tissue electrical conductivity by the use of MNPs, however, the local biochemical and bioimpedance effects of MNPs in cancerous tissue remain unknown, as a first approach to explore such effects caused by MNPs, the present work was focused on to assess biochemically the bioconjugated “MNPs - anti-HER2” systemic infusion effect on tumor tissue through studying the protein dynamics of the Growth Factor Receptor-Bound Protein 2 (GRB2) and its implication on the HER2 signaling pathway as well as its correlation with changes in bioimpedance measurement. A breast cancer model in rats in three specific conditions was evaluated by tissue immunostaining and bioimpedance measurements. Normal breast tissue (Healthy), Breast Cancer tissue (BC) and Breast Cancer tissue with MNPs (BC+MNPs). The results show that GRB2 protein expression in BC+MNPs is null or similar to Healthy, both compared with respect to BC. In addition, bioimpedance measurements are similar in BC+MNPs and Healthy conditions, and different values are evident in BC, those findings are in agreement with GRB2 expression. It seems that systemic bioconjugate infusion inhibits HER-2 signaling pathway and changes in the electrical conductivity of the tumor tissue.
Keywords: GRB2, Cancer, Breast, HER2, Nanoparticles
IOD, Integrated Optic Density; ANOVA, Analysis of Variance; PTM, Postransductional Modification; FGFR, Fibroblast Growth Factor Receptor
Breast cancer is a malignant uncontrolled proliferation that is mostly originated in the epithelial lining of large-sized or intermediate ducts (ductal) or terminal ducts of the lobules (lobular).1 25-30% of breast cancers show overexpression of HER-2 protein in tumor cells whose mutated forms promote neo plastic transformation of the cells, transmitting growth signals from the membrane to the nucleus increasing cell division since the ras-MAPK (mitogen activated mitogen-activated protein kinase) pathway is implied, and it’s implicated in cell proliferation.2-3 Overexpression of HER-2 is considered a bad prognostic factor because it is linked to a faster spread of tumor cells with an aggressive behavior.4 It also has an increase in angiogenesis, tumor invasiveness and apoptosis are decreased in this subtype.5
HER2 has tyrosine kinase activity, however, it has no known ligands, but is activated due to its overexpression, not when the expression is very low. Once the activation occurs, there is dimerization and stabilization of receptors which makes them get auto phosphorylated in their tyrosine residues, allowing the recruitment of adaptor proteins such as GRB2.6-8 This adaptor protein bounds in its SH3 domain, to an exchange of nucleotides called SOS (Son of Sevenless), which exchanges a Guanosine diphosphate (GDP) joined to ras, which gives it an inactive conformation, by a Guanosine triphosphate (GTP) which activates this pathway and triggers the MAPK, a kinase cascade.9
GRB2 is a small G protein with a weight of 25kd, widely expressed in almost all tissue, but with larger expression in brain, spleen, lungs and gastrointestinal tract, whose full sequence consists of a single SH2 domain flanked by two SH3 domains.10 In the ras-MAPK pathway, the SH2 domain mediates the association between GRB2 and HER2 receptor and it is strictly dependent on the autophosphorylation of the receptor.
Radiofrequency ablation reached importance in recent years for treatment of cancerous tumors. Ablation therapy of tumors by hyperthermia is an application of magnetic nanoparticles, since they have the capacity to produce heat in response to application of external magnetic fields.11 It is one of the procedures that have gained importance in recent years for the focal destruction of cancerous tumors in general, originally used in the treatment of liver tumors.12 Its clinical potential has expanded significantly in the treatment of breast cancer; however, experimental results of several research groups indicate that the effectiveness of the RFA in the treatment of cancer appears to be associated with the potential ability to focus electromagnetic energy in the specific cancerous zone.12-14
One of the treatments for breast cancer disease includes the targeted therapy with monoclonal antibodies (mAb). It is currently possible to covalently couple these antibodies to metal materials such as those that comprise the nanoparticles, so our research group proposed the idea of using Fe3O4 magnetic nanoparticle (MNPs) coupled to a monoclonal antibody anti-HER2 to generate a bioconjugated (MNPs - anti-HER2) in order to mark cancer cells with overexpression of the HER2 receptor selectively to increase the electrical conductivity of the cells and then be able to perform a selective ablation of tumor tissue, using RF energy.15-17 As far as the authors know, the effect of the “MNPs - anti-HER2” in the ras-MAPK pathway as well as its correlation with changes in tissue bioimpedance measurement is still unknown, so the aim of this study was to assess the GRB2 dynamics and tumor tissue bioimpedance measurements as a function of systemic infusion of the “MNPs - anti-HER2” in an experimental breast cancer model in rats.
Experimental design
To assess the GRB2 dynamics and tumor tissue bioimpedance measurements as a function of systemic infusion of the “MNPs - anti-HER2” in BC tissue, a breast cancer model in rats in three specific conditions (Normal breast tissue “Healthy”, Breast Cancer tissue “BC” and Breast Cancer tissue with MNPs “BC+MNPs”) were evaluated by tissue immunostaining and bioimpedance measurements. The Healthy condition was integrated by evaluations in the contralateral side of the cancerous zone in such a way that every experimental subject represents its own control.
Preparation of the bioconjugated “magnetic nanoparticles-anti-HER2” and tumor induction
The implementation process of the bioconjugated was made following the carbodiimide covalent coupling method (A10 protocol Chemicell) using Nano-screenMAG-ARA nanoparticles with matrix of glucuronic acid and mouse monoclonal antibody anti-HER-2. For tumor induction, it was used N-methyl-nitrosourea (MNU) + isotonic solution with pH adjustment to 5. It was injected via intraperitoneal to a batch of 35 rats at a dose of 50 mg/kg and they were explored weekly to check tumor induction.18-20 5 rats with mammary tumors were identified; those were distributed in groups in the following way: 2 rats in the BC group and 3 rats in the BC+MNPs group.
Bioconjugated administration
Both groups went under general anesthesia, a catheter was placed in the jugular vein, and we administered the bioconjugated to the BC+MNPs group, at a dose of 40 mg/kg for 15 minutes, and a magnet was placed in the tumor region to ensure that the highest concentration of the bioconjugated was found in the area of interest. The BC group was infused with isotonic solution, and a magnet was placed in the tumor region (Figure 1). At 24 hours after infusion, a surgical excision of tumors was made.
Figure 1 Bioimpedance measurments in tumoral tissue (A) and contralateral healthy tissue (B) 24 Hs after bioconjugate infusion.
Collection and sample preparation
Sequential cuts of tissue fixed in formalin and embedded in paraffin were made, and then stained for GRB2 as described following subsection. Approval of the institutional Committee on the care and use of laboratory animals (CICUAL) (Dictamen 04-22-02-12) and of the Committee of Ethics at the ¨Escuela Superior de Medicina-Instituto Politécnico Nacional¨ (Dictamen 01-22-02-12).
Immunohistochemistry stains method
The staining method for the protein GRB2 was an indirect Immunohistochemistry. For Antigen retrieval, cuts were immersed in Citrate buffer pH 6. They went into a preheated pressure canner and were subjected to steam pressure for 5 minutes, after that the samples where placed in a hydrogen peroxide 30% solution in order to block the endogenous peroxidase. Then, samples were incubated with normal serum and subsequently with the primary antibody anti-GRB2 (1:100) (Rabbit IgG Polyclonal Antibody anti-GRB2 and InmunoDetector Protein Blocker / Antibody Diluent for 60 min at room temperature and then washed with saline buffer TRIS (TBS) Auto Wash Buffer, 40 X. Used an IgG anti-rabbit coupled to HRP secondary antibody in incubation for 30 minutes and revealed with diaminobenzidine. They were counterstained with Mayer´s hematoxylin, and after the tissue was dehydrated, it was mounted. Anti-rabbit IgG (Rabbit negative control), was used in a sample, as a negative control.
Immunofluorescence stains method
The staining method for the protein GRB2 was an indirect Immunohistochemistry. For Antigen retrieval cuts were immersed in Citrate buffer pH 6. They went into a preheated pressure canner and were subjected to steam pressure for 5 minutes. It was permeated with Triton 1% + phosphates buffer for 10 minutes at room temperature. Then incubated with bovine albumin 2% for 1 hour and then samples were incubated with the primary antibody anti-GRB2 (1:100) overnight at 4°C and were then washed with phosphate to incubate secondary antibody for 60 minutes and with E-cadherin 60 minutes more. Finally, the samples were counterstained with DAPI for 10 minutes and then the coverslip was mounted with Antifade mounting medium.
Microscopy and acquisition of digital images
The histological sections were displayed on an epifluorescence microscope (Nikon, Microphot FXA) with a magnifying power of 8 X and 40 X camera Nikon DXM1200F). The acquisition of images was performed with the ACT-1, Nikon software.
Bioimpedance measurements
Bioimpedance measurements were developed by a high precision impedance analyzer, the device uses an alternating current I cos(ωt), in the frequency range of 100 MHz. First; measurements were developed in tumor as well as in its contralateral healthy tissue, then; bioconjugated was infused systemically in the BC+MNPs group as described in subsection above, finally; bioimpedance measurements were developed as previous description after bioconjugated infusion.
Chemicals and Reagents
Instruments
Results
GRB2 predominates in stromal cells in breast cancer tissue samples without bioconjugated: GRB2 breast tissue distribution in the BC+MNPs group was quantitatively evaluated, and so we found it predominantly in stromal cells, and not so in the mammary ducts. This was different from the findings in the BC group and the Healthy breast tissue group, as it is shown in Figure 2.
GRB2 expression and Bioimpedance values increase in breast cancer samples without bioconjugated and decrease in the protein expression in the samples with bioconjugated: The protein expression of GRB2 was determined by immunohistochemistry, and the analysis of its expression was made through a digital image analysis with the fields obtained from the samples where the values of the integrated optic density (IOD) were obtained, and so we found that the breast cancer samples (BC) showed a higher IOD compared to that shown in the Healthy tissue samples. This observation is consistent with the direct relationship between the overexpression of HER2 and GRB2. However, in the BC+MNPs, the obtained IOD was considerably lower than in the BC group, with similar values to those obtained from the normal breast samples (Healthy). Bioimpedance measurements are in agreement with GRB2 expression, similar bioimpedance values in BC+MNPs and Healthy conditions, and higher values are evident in BC, those findings indicate an electrical conductivity increase in BC tissue given the presence of MNPs.

Figure 2 Comparison of the GRB2 protein expression in normal breast tissue (Healthy) and breast cancer tissue without bioconjugate (BC) and with bioconjugate (BC+MNPs). The specific signal is shown in Brown (counterstained hematoxylin. 8 X (left)) 40 X (right); bar, um 20).
A statistical analysis was made using the Kruskal-Wallis ANOVA test, and the Mann-Whitney U test for independent groups, through the software “Statistics 7.0”; this test clearly shows a difference between the samples of the BC+MNPs and the samples of the BC group. The results were confirmed by quantitative immunofluorescence and bioimpedance values (Figures 3 & 4).
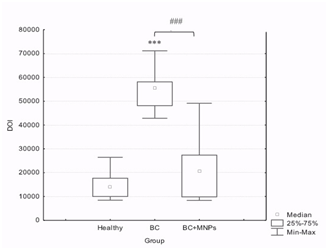
Figure 3 Semiquantitative comparison of the GRB2 protein expression as a function of IOD in normal breast tissue (Healthy), breast cancer tissue without bioconjugate (BC) and with bioconjugate (BC+MNPs). A Kruskal-Wallis ANOVA Test showed significative diferencees among groups (P< 0.0001) and a Mann-Whitney U test showed diferences betwen groups (P< 0.0001)*** with regard to its normal control, ### as indicated.
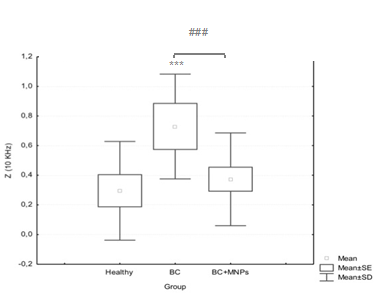
Figure 4 Bioimpedance measurements comparision in normal breast tissue (Healthy), breast cancer tissue without bioconjugate (BC) and with bioconjugate (BC+MNPs). A Kruskal-Wallis ANOVA Test showed significative diferencees among groups (P< 0.0000) and a Mann-Whitney U test showed diferences betwen groups (P< 0,041322)*** with regard to its normal control, ### as indicated.
Discussion
Ductal GRB2 expression in breast cancer tissue belonging to the BC+MNPs was practically null and similar to the observation in Healthy condition, in contrast to the expression obtained in BC tissue. This result was obtained by both immunohistochemistry and immunofluorescence. So far it was known that the GRB2 protein plays an important role in many signaling pathways and that it is located in many tissues, including the embryonic.21 Also, that the degree of Association of HER-2 with GRB2 is proportional to the expression of HER-2 in breast tumors .22 however, literature does not mention of the nature of this protein. As an adaptor protein, when the tyrosine kinase receptor gets phosphorylated, GRB2 is recruited from the cytoplasm to the cell inner membrane, so we initially considered the possibility that the presence of the bioconjugated in the breast cancer tissue with HER2 overexpression would prevent dimerization of the receptor with others from the same family and that therefore it would not get phosphorylated, and GRB2 would not be recruited to the membrane however, our results show that the presence of the bioconjugated does not affect the GRB2 dynamics, but its presence in the epithelial cells of the mammary ducts, since it was not observed in these cells or had a very similar expression of that in the normal breast tissue. We assume that the possible explanations for this observation are as follows: GRB2 is an inducible protein and not a constitutive one, or GRB2 undergoes a postransductional modification (PTM) at the epitope site. For the first explanation, there is the possibility that the bioconjugated is indeed joining the dimerization site of the HER2 receptor and therefore autophosphorylation not occurring, so it would not need a scaffold protein for the ras-MAPK signaling pathway, being reflected in the absence of transcription of GRB2 (Figure 5). Such an assumption could explain the fact that the protein was found in stromal cells, where its presence does not depend on the expression of HER2, but where it is involved in the signaling pathway mediated by the Fibroblast Growth Factor Receptor (FGFR); these cells are the most abundant in the stroma. About the other possibility, it is known that protein can get phosphorylated in specific situations.23 and it was also found recently that it might present SUMOylation.24 Still, other PTM or the impact they may have on the signaling pathways have not been studied thoroughly. However, it is known that both phosphorylation and SUMOylation of GRB2 act as regulatory mechanisms of signaling, in different ways. GRB2 Tyrosine phosphorylation (Y) 209 decreases the binding of the SOS protein SH3 domain, so the mitogen signal level of ras pathway decreases. On the other hand, the SUMOylation of the lysine (K) 56 promotes the formation of a complex GRB2-SOS increasing the signalization of the same pathway. Interestingly, this modification occurs at the junction of the SH2 and SH3 protein domains, the site recognized as an epitope for some antibodies anti-GRB2. Therefore, consider that the presence of the bioconjugated could induce a PTM of the GRB2 protein in certain organisms, is a possible explanation for the lack of observation of the same, in Figure 6 we proposed a possible HER2 pathway inhibition as a function of the bioconjugated infusion. Bioimpedance measurements reflect changes in the beta dielectric dispersion range, specifically at 10 KHz, where structural changes at cells structure level are expected. The bioimpedance observations are in agreement with GRB2 expression, similar bioimpedance values in BC+MNPs and Healthy conditions, and higher values are evident in BC, those findings indicate an electrical conductivity increase in BC tissue, such as expected given the characteristic vascularity increased in cancer, in contrast, changes in the structural cells given the presence of nanoparticles in the BC+MNPs might be associated to the diminishing bioimpedance observed.
The poor observation of GRB2 in the ductal cells under the bioconjugated presence suggest two metabolic possibilities: The bioconjugated inhibit the signaling pathway and therefore the GRB2 protein is an inducible protein rather than a constitutive one, or the bioconjugated induces a postransductional modification in the GRB2 epithope site that enables the antibody´s recognizing. The observation of GRB2 in the stromal cells suggest that its presence there, it is not determined by the GRB2 signaling pathway. In addition; the systemic bioconjugated infusion change the electrical conductivity of the tumor tissue at beta dielectric dispersion frequencies, it represents changes in the structural cells given the presence of MNP´s and thus diminishing the bioimpedance of the cancerous region. This study represents a first approach with the purpose of knowing the possible effect caused by the bioconjugated “MNPs - anti-HER2” systemic infusion on tumor tissue, and further studies to determine the specific metabolic signaling pathways affected and confirm the observations are warranted.
This work was developed in the Laboratory of Physiology of the “Escuela Militar de Graduados de Sanidad-Universidad del Ejército y Fuerza Aérea” (EMGS-UDEFA) and the Oxidative Stress Laboratory “Sección de estudios de posgrado de la Escuela Superior de Medicina del Instituto Politécnico Nacional”. Was supported by “CONACYT CB-2012” under the grant no. 180536. We are also very grateful to Dr. Ana Alfaro for the expertise advice.
None.

©2017 Alvarez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.