Journal of
eISSN: 2377-4282


Review Article Volume 3 Issue 4
1Ave Real de Haciendas S/N. Aguascalientes, Mexico
2Universidad de Oriente, Venezuela
3Departamento de Quimica, Universidad de Oriente, Venezuela
4Instituto Tecnologico de Aguascalientes, Mexico
Correspondence: Norma Aurea R Vazquez, PCC.Ave Real de Haciendas S/N. Aguascalientes, México, Tel: 4566156465 , Tel 4566156465
Received: January 28, 2016 | Published: April 21, 2016
Citation: Vazquez NAR, Alexander EMB, Ricardo R, Francisco-Javier SR (2016) Analysis Spectroscopy of the Absorption of Calcium Carbonate on Graphene/Polyurethane Composites Applied Computational Simulation and Artificial Neuronal Networks. J Nanomed Res 3(4): 00061. DOI: 10.15406/jnmr.2016.03.00061
One of the most important applications of graphene is in the field of polymer nanocomposites, where graphene is introduced as filler with the aim to improve their chemical and physical properties. Such as polystyrene (PS), PMMA, PVA, polypropylene (PP), epoxy, polyester, silicon foams, polyurethanes (PU) and polycarbonates (PC), graphene-based nanomaterials have been utilized in a myriad of bioapplications (e.g., drugs and gene delivery, nanomedicine, bioimaging and potential cancer therapies). The graphene, Polyurethane (PU), calcium carbonate (CC) and absorption of CC in graphene/PU composite, respectively were studied using theoretical calculations like Gibbs free energy, log P, Fourier Transform Infrared (FTIR) and electrostatic potential. FTIR spectroscopy reveals information about the molecular interactions of chemical components and is useful for characterization of composite. Nucleophilic and electrophilic regions were calculated using the electrostatic potential. It was determined that the hydrophilic (log P, octanol–water distribution constant) values play an important role in the absorption process of CC.
Keywords: Graphene, Polyurethane, Modeling, Composites
PS, Polystyrene; PP, Polypropylene; PU, Polyurethanes; PC, Polycarbonates; CC, Calcium Carbonate; FTIR, Fourier Transform Infrared; WPU, Waterborne Polyurethane; FGS, Functionalized Graphene Sheet; TPU, PU-Thermoplastic; AM1, Austin Model 1; ANNs, Artificial Neural Networks; SOM, Self-Organization Map; BP, Backward Propagation
The field of nanoscience has blossomed over the last two decades and the importance of nanotechnology increase in areas such as computing, sensors, biomedical and many other applications. In this field the discovery of graphene and graphene–based polymer nanocomposites is an important addition in the field of nanoscience. The physicochemical properties of the nanocomposite depend on the distribution of graphene layers in the polymer matrix as well as interfacial bonding between the graphene layers and polymer matrix.1 Graphene can be described as a one-atom thick.2 layer of graphite. It is the basic structural element of other allotropes, including graphite, charcoal, carbon nanotubes and fullerenes.
It can also be considered as an indefinitely large aromatic molecule, the limiting case of the family of flat polycyclic aromatic hydrocarbons.3-5 The composites with graphene have been prepared with a number of polymers, mainly polyurethane (PU), which can provide properties covering from a high performance elastomer to tough thermoplastic with diexcellent physical properties, including high tensile strength, abrasion and tear resistance and solvent resistance. In addition, its high versatility in chemical structures originated from a wide range of monomer materials affords tailor–made properties with well–designed combinations of these monomers. As a result, PU can be easily manipulated to satisfy the highly diversified demands of modern technologies.
In order to decrease emissions of volatile organic compounds, the development of environmentally friendly waterborne polyurethane (WPU) has been increasing, especially in the field of coating industry where the reduction of evolution of volatile organic compounds during the drying process is critical. In addition, WPU offers many advantages such as low viscosity at high molecular weight and good applicability, which cannot be realized with conventional solvent–borne systems .6 Lee et al.7 prepared water-borne polyurethane (WPU)/functionalized graphene sheet (FGS) nanocomposites by in situ method. The electrical conductivity of the nanocomposite was increased 105–fold compared to pure WPU due to homogeneous dispersion of FGS particles in WPU matrix. The presence of FGS also increased the melting temperature and heat of fusion of the soft segment of WPU in the nanocomposites.
Liang et al. 8 prepared three types of nanocomposites by solution mixing process. They used isocyanate modified graphene, sulphonated graphene and reduced graphene as nanofiller and PU-thermoplastic (TPU) as the matrix polymer. The rate of thermal degradation of TPU/isocyanate modified graphene nanocomposites is much higher than that of TPU/sulphonated graphene and TPU/reduced graphene nanocomposites. There has been a surge of interest in developing graphene for drug loading and delivery because strong interaction exists between hydrophobic drugs and aromatic regions of graphene sheets. Dai’s group initially developed NGO–PEG (nanographene oxide–polyethylene glycol) as a nanocarrier to load variant anticancer drugs via nancovalent physisorption and evaluated it’s in vitro cellular uptake capacity and photo luminescent property.9-10
Quantum chemical calculations produce a large amount of output data. Therefore it is important to decide which output is elevated for the chemical, biochemical, or biological mechanism underlying the phenomenon under investigation.11 The PM3 semi empirical method (Parameterized Model number 3), is selected (the Hyperchem software, release 7.0, being used). This method can automatically optimize the bond length, angle and twist angle and yield a lot of information on the structure; define and select the output parameter. PM3 uses a Hamiltonian that is very similar to the AM1 (Austin Model 1) Hamiltonian but the parameterization strategy is different. While AM1 was parameterized largely based on a small number of atomic data, PM3 parameterized to reproduce a large number of molecule´s properties. In some sense, chemistry gave way to statistics with the PM3 model. Different parameterization and slightly different treatment of nuclear repulsion allow PM3 to treat hydrogen bonds rather well but it amplifies non–physical hydrogen–hydrogen attractions in other cases. This results in serious problems when analyzing intermolecular interactions (methane is predicted to be a strongly–bound dimmer) or conformations of flexible molecules (OH is strongly attracted to CH3 in 1–pentanol). The accuracy of thermochemical predictions with PM3 is slightly better than of AM1. The PM3 model has been widely used for rapid estimation of molecular properties and has been recently extended to include many elements, including some transitions metal.12-13
In the past decade, artificial neural networks (ANNs) have received a great deal of attention among scientists and engineers and they are being touted as one of the greatest computational tools ever developed. Much of this excitement is due to the ability of neural networks to emulate the brain’s ability to learn by example. Specifically, ANNs are known to be a powerful tool to simulate various non-linear systems and have been applied to numerous problems of considerable complexity in many fields, including engineering, psychology, medicinal chemistry, diagnostics and pharmaceutical research.14 The application of ANNs in the field of pharmaceutical development and optimization of the dosage forms has become a topic of discussion in the pharmaceutical literature.15
The potential applications of ANNs methodology in the pharmaceutical sciences are broad as ANNs capabilities can be summarized by modeling, pattern recognition and prediction. Thus, applications of ANNs include drug modeling, dosage design, protein structure and function prediction, pharmacokinetics and pharmacodynamics modeling, as well as, interpretation of analytical data and in vitro/in vivo correlations.14
In this project the study of absorption process of CC was realized using PM3-semiempirical method for determine ΔG, structural parameters, FTIR and MESP of PU, Graphene, PU/Graphene composite and through data of bond lengths, bond angles and energies, the calculous were compared with ANNs for verified the absorption process.
Geometry optimization
In this study the semiempirical method was used for describing the potential energy function of the system. The optimizing process of structures used in this work carried out using the PM3 method, because it generates a lower-energy structure even when the initial structure is far away from the minimum structure. The Polak–Ribiere algorithm was used for mapping the energy barriers of the conformational transitions. For each structure, 1050 iterations, a level convergence of 0.001kcal mol Å and a line search of 0.1 were carried out.16
Structural parameters
The optimized structural parameters were used in the vibration wavenumber calculation with PM3 method to characterize all stationary points as minima. The structural parameters were calculated select the Constrain bond and length options of Build menu for two methods of analysis.
FTIR
The FTIR was obtained by first selecting menu Compute, vibrational, rotational option, once completed this analysis, using the option vibrational spectrum of FTIR spectrum pattern is obtained from the method of analysis.17
Electrostatic potential
After obtaining a Gibbs free energy or optimization geometry using the PM3 method, we can plot two–dimensional contour diagrams of the electrostatic potential surrounding a molecule, the total electronic density, the spin density, one or more molecular orbitals and the electron densities of individual orbitals. Hyperchem software displays the electrostatic potential as a contour plot. Choose the values for the starting contour and the contour increment so that you can observe the minimum (typically about –0.5 for polar organic molecules) and so that the zero potential line appears.18
Artificial neuronal networks (ANNs)
One of the more important ANN is the Self-Organization Map (SOM) proposed by Kohonen. In this network there is an input layer and the Kohonen layer, which is usually, designed as two-dimensional arrangement of neurons that maps n-dimensional input to two-dimensional. It is basically a competitive network with the characteristic of self-organization providing a topology-preserving mapping from the input space to the clusters. Mathematically networks and SOM require input vectors xJ=(x1,x2,x3,.....xJP) training weights wl=(wl1,wl2,.....wlp), where l indicates the number of nodes, Wlj denote the weights assigned to entry of nodeand is number input variables.where l indicates the number of nodes, lj w denote the weights assigned to entry j x of node l and p is number input variables One way to establish the structure of the SOM type neural network is shown in Figure 1, which indicates that the basic structure of the network must be input layer, hidden layer and output layer, the number of neurons in each layer is determined by the information processing capacity and robustness to want to project the problem, the problem presented in this work a static neural network is established with a number finite neurons, 20 neurons in each of the layers of the neural network.
The distance between each of the nodes is made using the equation of Euclidean distance between the input nodes and the projection (bond lengths), Eq (1)
ANNs must be trained, to establish minimum and maximum parameters of the projection of the bond lengths, usually workouts rate applied backward propagation (BP), for study case, a training method type is batch learning, Eq (2).
Where α denotes function of excitation each neuronal network, e error of propagation is training which should tend to zero, Nis total number of nodes in the neuron network. The use of batch mode training provides an accurate gradient vector, thus ensuring convergence to a local minimum, this basically does not happen with sequential training as BP because it becomes a search in the space of weights estimation stochastically, however the stochastic nature prevents convergence of the algorithm.
The set of thermodynamic data obtained is listed in Table 1.The negative value of(Gibbs free energy) reflects the spontaneity of materials. Attractive interactions between
Properties |
IPN/CC |
ΔG (kcal mol-1) |
-4173.19 |
Log P |
-1.87 |
Table 1 QSAR properties for composite
Structural parameters
Computational analysis was carried out to determine the optimized geometry of graphene, PU, CC, respectively. Table 2 shows the results obtained by ANNs, as discussed the previous literature, the changes in frequency or bond length of the C–H bond on substitution is due to a change in the charge distribution on the carbon atom of the ring. The substitutes may be either of the electron withdrawing type (Cl, Br, F, etc.) or electron donating type (CH3, C2H5, etc.). The reverse holds well on substitution with electron donating groups. The actual change in the C–H bond length would be influenced by the combined effects of the inductive–mesmerize interaction and the electric dipole field of the polar substitute. The calculated geometric parameters can be used as foundation to calculate the other parameters for the compound.23
Bond |
ANNs (Å) |
Bond |
ANNs (Å) |
Bond |
ANNs (Å) |
Bond |
ANNs (Å) |
PU |
Graphene |
CC |
|||||
O9=C8 |
1.157 |
C1-C2 |
1.4932 |
C28-C29 |
1.4951 |
1st mole |
|
C8=N1 |
1.2193 |
C2-C3 |
1.4543 |
C29-C30 |
1.5338 |
Ca1-O2 |
2.383 |
N1-C2 |
1.4581 |
C3-C4 |
1.4546 |
C30-C16 |
1.4933 |
O3-C4 |
1.4284 |
C2-C3 |
1.6203 |
C4-C5 |
1.4546 |
C25-C24 |
1.4551 |
C4-O2 |
1.4284 |
C3-C4 |
1.5394 |
C5-C6 |
1.4929 |
C24-C23 |
1.4518 |
O2-Ca1 |
2.383 |
C4-C5 |
1.6067 |
C6-C1 |
1.5338 |
C23-C22 |
1.4571 |
C4=O5 |
1.2176 |
C5-C6 |
1.6201 |
C4-C7 |
1.4568 |
C22-C13 |
1.4577 |
|
|
C6-C7 |
1.6228 |
C7-10 |
1.4568 |
C22-C21 |
1.4557 |
2nd mole |
|
C7-N10 |
1.5247 |
C10-C9 |
1.4546 |
C21-C20 |
1.4557 |
Ca1-O2 |
2.383 |
N10-C11 |
1.4288 |
C9-C8 |
1.4921 |
C20-C19 |
1.4578 |
O3-C4 |
1.4284 |
C11=O13 |
1.2276 |
C8-C5 |
1.4921 |
C20-C32 |
1.4571 |
C4-O2 |
1.4284 |
C11-O12 |
1.3713 |
C10-C17 |
1.4545 |
C32-C31 |
1.4517 |
O2-Ca1 |
2.383 |
O12-C15 |
1.4051 |
C17-C16 |
1.4543 |
C31-C28 |
1.4551 |
C4=O5 |
1.2176 |
C15-C16 |
1.6301 |
C16-C15 |
1.4932 |
C24-C40 |
1.4943 |
|
|
C16-C17 |
1.6083 |
C15-C14 |
1.5338 |
C40-C39 |
1.5346 |
3rd mole |
|
C17-C18 |
1.5384 |
C14-C9 |
1.4929 |
C39-C38 |
1.5345 |
Ca1-O2 |
2.383 |
C18-C19 |
1.6044 |
C2-C27 |
1.4933 |
C38-C37 |
1.4921 |
O3-C4 |
1.4284 |
C19-C20 |
1.6077 |
C27-C26 |
1.5337 |
C37-C23 |
1.4526 |
C4-O2 |
1.4285 |
C20-O21 |
1.4043 |
C26-C25 |
1.4947 |
C37-C36 |
1.4923 |
O2-Ca1 |
2.383 |
O21-C22 |
1.3658 |
C25-C12 |
1.4538 |
C36-C35 |
1.4923 |
C4=O5 |
1.2176 |
C22=O25 |
1.2272 |
C12-C3 |
1.4539 |
C35-C21 |
1.4554 |
|
|
C22-N23 |
1.4071 |
C12-C13 |
1.4566 |
C35-C34 |
1.4924 |
|
|
N23-C26 |
1.4506 |
C13-C11 |
1.456 |
C34-C33 |
1.4924 |
|
|
C26-C27 |
1.6533 |
C11-C7 |
1.4565 |
C33-C32 |
1.4526 |
|
|
C27-C28 |
1.822 |
C11-C19 |
1.456 |
C33-C43 |
1.4922 |
|
|
C28-C29 |
1.6525 |
C19-C18 |
1.4566 |
C43-C42 |
1.5349 |
|
|
C29-C30 |
1.5408 |
C18-C17 |
1.454 |
C42-C41 |
1.5349 |
|
|
C30-C31 |
1.6206 |
C18-C28 |
1.4539 |
C41-C31 |
1.4945 |
|
|
C31-N32 |
1.4582 |
|
|
|
|
|
|
N32=C33 |
1.2194 |
|
|
|
|
|
|
C33=O34 |
1.1571 |
|
|
|
|
|
|
Table 2 Comparation of structural parameters of graphene/PU/CC composite using PM3 and ANNs.
Table 3 shows the FTIR results of absorption of CC in graphene/PU composite. The results show an appreciable difference of the different materials alone with respect to composite, indicating a strong interaction between the three materials.
Assignment |
Frequency (cm-1) |
CH2 symmetric vibrations (PU) |
5380–5261, 4424–4040, 3304 |
C=N symmetric vibrations (PU) |
3947, 3781–3709 |
NH (PU) |
3709, 3394 |
CH2 symmetric, C=O vibrations (PU) |
3518 |
C–C stretching (PU) |
3209, 2219 |
C=C (Graphene) |
3034, 2628, 2412 |
C–C (Graphene) |
2805, 1948, 1315, 1026, 733 |
C=O (PU) |
1735 |
C–N,O–C–O (PU) |
2219, 1678, 1026 |
O–C–O (PU) |
1678 |
C–O–Ca (CC) |
1507, 934 |
C–N, C-O (PU) |
611 |
Ca–H (CC–PU), C–H (Graphene–PU) |
61 |
Table 3 FTIR result of graphene/PU/CC
Electrostatic potential
Figure 2 demonstrates the MESP of absorption process of CC in graphene/PU composite and their electrostatic interactions, showing the negative (red) regions were related to electrophilic reactivity and the positive (blue) regions to nucleophilic reactivity. The absorption of CC by the graphene/PU has mainly involved the formation of hydrogen bonds between PU and graphene and CC with PU respectively. The negative log P values for the graphene/PU indicated that absorption properties of the composite are dominated by its hydrophilic character and that absorption plays an important role in the CC absorption partitioning and receptor–binding. It was also shown that graphene changes the hydrophobicity of PU. The MESP values from 0.0837 at 0.067 eV indicated that the nucleophilic and electrophilic regions mainly involved the C=O, C–O of the PU and CC, respectively so graphene molecule, respectively.
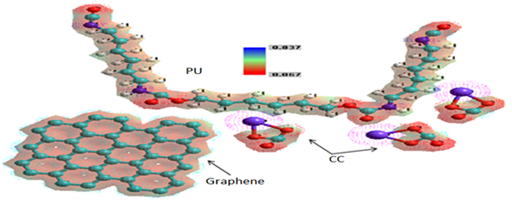
Figure 2 MESP of graphene/PU/CC, where: red color–oxygen, white color–hydrogen, light blue–carbon and dark blue–nitrogen atom, respectively.
Artificial Neuronal Networks (ANNs)
ANNs can be applied in the recognition of images, but not only are limited in that application; these also can be applied in the estimate or projection in the molecular simulation. The application of ANNs in molecular simulation is given by estimating possible formation of molecules this is achieved by applying the neural network SOM (Self -Organizing Map), which made possible a projection of the molecule on a space in two dimensions (2-D). A demonstrated the interaction between PU and graphene is added a molecule of CC, to make the introduction of CC is observed between the molecule CC and PU interaction exists Figure 3, graphene remains the molecule with higher proportion of link between similar molecules graphene but still present interaction between the PU, the above is shown in the bond lengths between the PU and CC (Figure 4).
None.
None.

©2016 Vazquez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.