Journal of
eISSN: 2377-4282


Mini Review Volume 7 Issue 2
1Department of Pathobiology and Veterinary Science, University of Connecticut, USA
2Department of Microbiology and Immunology, Al-azhar University, Egypt
Correspondence: Mazhar I Khan, Department of Pathobiology and Veterinary Science, University of Connecticut, Storrs, CT 06269, USA
Received: January 18, 2018 | Published: April 4, 2018
Citation: Li J, Helal Z, Khan MI. Self–assembling protein nanoparticles open a new avenue for next generation veterinary vaccines. J Nanomed Res. 2018;7(2):82 ?8 4 DOI: 10.15406/jnmr.2018.07.00181
Vaccines are one of the most outstanding innovations in public health. They save millions of lives by the prevention of infectious diseases. Throughout the past two centuries, most vaccines have been formulated by live or killed whole pathogens. Live vaccines have a safety issue that is a risk of reversing back to their virulent state. Kill vaccines have weak immunogenicity and are cumbersome to manufacture. Nanobiotechnology has emerged as a powerful alternative platform in vaccine design, which address the safety issue as well as enhancing the immunogenicity of vaccines. Self-assembling protein nanoparticles is one of the most promising platform (SAPN) at nanoscale. In this mini-review, we primarily focus on the discussion of the concept of nanovaccine and the principle of SAPN in vaccine development, and will highlight its applications in designing vaccines for veterinary use.
Keywords: self-assembling, protein nanoparticles, vaccines, avian, influenza, infectious bronchitis
Vaccines have been implemented for more than two centuries and have saved millions of lives, which is one of the most outstanding inventions in medicine. The concept of vaccine was conceived two centuries ago by the English physician Edward Jenner, “the father of immunology”. He had initiated the first protective vaccine against smallpox using live cowpox virus in 1798. Many effective vaccines have been developed in the past two hundred years. The majority of them was produced by traditional methods by attenuating live pathogens or by chemically inactivate whole pathogenic organisms. Live attenuated vaccines are highly protective but bear inherent safety concern due to the potential reactivation of virulent state. In contrast, chemically inactivated vaccines cannot regain the virulent state of derivative pathogens and are safe. However, they are poorly immunogenic, and induce weak protection. Besides, tediously labor-intensive efforts are required for formulation preparation of killed vaccines.
The breakthrough progress of genetic engineering in the 1980s allows vaccinologists to address the safety concerns by developing subunit vaccines, which contain one or multiple antigens from a whole pathogen. In this mini-review, we will discuss the application of self-assembled protein nanoparticles in the development of safe subunit vaccines, and will highlight the current progress of its application in poultry vaccines.
Origination of the concept of nanovaccine and its advantages
The vaccine against Hepatitis B is the first successful application of the concept of a nanoparticle-based vaccine. The surface antigen of hepatitis B (HBsAg) was the first antigen synthesized and assembled into nanoparticles (NP) in yeast using recombinant DNA technology, which are similar to 22 nm virus-like particles (VLP) secreted by infected human cells.1 The genetically engineering vaccine against Hepatitis B was licensed in 1986.2 Since then, the concept of nanoparticle vaccine or nano scale vaccine has been expanded to the design of immunogens using a wide range of carrier materials at nano scale from 1–1000nm, which include VLPs, virosomes, liposome, emulsion, polymer-copolymer NP, viral vector, immune-stimulating complex (ISCOM) and self-assembling protein nanoparticle.3–7
NP vaccines are safe due to their non-replicable nature. They can be easily engulfed by antigen presenting cells because they have well-defined shape and size resembling a virus particle.8,9 NP is typically constructed using a repetitive building scaffold. Thu, NP vaccines have repetitive epitopes on their surfaces. This feature constitutes a multivalence, which is able to cross-link B cell receptors and results in the maturation of naïve B cells.10 In addition, NP vaccines have also been demonstrated induces memory cytotoxic T cell responses against malaria via cross-presentation, which was associated with antigen-associated protein in early endosomes.11 Most importantly, NP vaccines have demonstrated to be self-adjuvanted by co-displaying immune-stimulatory molecules.12,13 Therefore, the application of NP vaccines offers an excellent solution to the long-standing issue of safety versus immunogenicity.
Principle of SAPN as vaccine platform
Among different categories of NP vaccines, self-assembled protein nanoparticles (SAPN), based on coiled-coil folding domains, have emerged as one of most appealing tools in vaccine design. The α-helical coiled-coils are highly versatile protein oligomerization domains characterized by seven-residue repeats called heptad repeats (Figure 1A). Apolar residues preferentially occur in the first (a) or in the fourth (d) position within a unit of heptad repeat whereas the fifth (e) or the seventh (g) position prefers charged residues. A linear peptide chain that containing heptad repeats is able to self-assemble into a left-handed α-helix driven by intramolecularly hydrophobic interaction between ‘a’ and ‘d’ apolar residues (Figure 1A). The α-helix is also stabilized by intramolecular ionic interaction between ‘e’ and ‘g’ charged residues. Coiled-coils consist of two to five α-helices and can self-assemble into supercoils via intermolecular ‘knobs-into-holes’ interaction to form a hydrophobic core, in which the quaternary structure of supercoils is stabilized by intermolecular ionic interactions between side chains of charged residues (Figure 1B).14–16 This is the principle of oligomerization of coiled-coil domains, which could be used to produce nanoparticles.17 It was demonstrated by Peter Burkhard group that chimeric peptides containing two coiled coils connected by a short linker region that are capable of self-assembling into a roughly spherical nanoparticle with a regular symmetry. They reported that 60 monomeric peptide chains containing two chimeric coiled-coil domains of two or three and five α-helices were able to self-assemble into nanoparticles with dodecahedron/icosahedron symmetry. The resultant nanoparticles had a size about 16nm~ 25nm, which resemble a virus capsid.17 This platform was demonstrated to be a well-suited system for vaccine delivery against infectious diseases. B cell epitopes could be linked to two both ends of chimeric coiled-coil folding domains in a monomeric peptide chain. SAPN allows the repetitive presentation of oligomeric antigens on the NP surfaces in their native conformations, by which the oligomerization state of the epitopes is determined by the oligomerization state of their adjacent coiled-coils.18 This well-understood toolbox has been used to successfully generate prototypic vaccines against SARS, HIV, and Malaria. In the study of prototypic SAPN vaccine against SARS, they demonstrated mice immunized with SAPN displaying coiled-coil epitope from the spike protein of SARS virus eliciting conformation-specific neutralizing antibodies that recognizing the α-helical heptad repeat region at the C terminus of the Spike protein.19 SAPN was also used to display conformation-specific 4E10 and 2F5 epitopes of HIV.20 A T helper cell epitope (PADRE) could also be engineered into the trimerization domain and a self-adjuvanted SAPN for a malaria prototypic vaccine. Robust and long lasting humoral and cellular immune responses against malaria were induced by B and cytotoxic T cell epitopes, which were co-expressed with a pentamerization and a trimerization domain at the N and C terminus, respectively.12,21
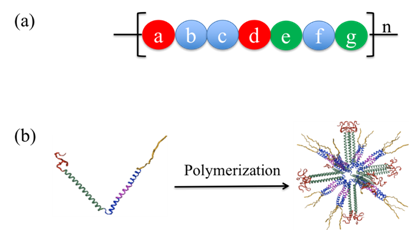
Figure 1 (A) A heptad repeat unit. Amino acid residues occupying a heptad repeat are denoted with a, b, c, d, e, f and g. Position ‘a’ and ‘d’ (in red) are usually occupied by hydrophobic amino acids. Position ‘e’ and ‘g’ (in green) are charged amino acids. Position ‘b’, ‘c’ and ‘f’ are random amino acids. (B) cartoon shows the principle of polymerization of monomeric peptide chains into nanoparticle, which is dictated by hydrophobic and ionic interactions among amino acid residues in position ‘a’ and ‘d’, and position ‘e’ and ‘g’, respectively. Image was adopted from Keba et al.21
Application of SAPN in veterinary vaccines
Most of the market available veterinary vaccines are live or killed whole organism vaccines. Although they are effective against diseases homologous to antigens contained in the vaccines, they have some other drawbacks. For instance, they are less effective to diseases mismatching to vaccine antigens, especially highly mutable emerging diseases like avian influenza (AI) and infectious bronchitis (IB); they require cumbersome labor to culture pathogens in cell cultures or pathogen-specific eggs, by which is expensive and the production period could be up to a year; importantly, it’s difficult to differentiate infected from vaccinated animals (DIVA) when these conventional vaccines are applied because they interfere the surveillance diagnosis with serological methods. This will compromise the international trade of a country producing animal products free of a disease.22
The successful applications of SAPN in vaccine design further demonstrate that SAPN could be potential alternatives to current live and killed vaccines for veterinary usage. It is relatively safe and could be formulated and scaled up in a short period of time using a prokaryotic expression system. Here, we highlight current progress in the application of SAPN in designing vaccines against avian influenza and infectious bronchitis, two highly contagious respiratory diseases in poultry that cause enormously economic losses. A SAPN formulation for avian influenza was reported that ectodomain of matrix protein 2, a conserved sequence of influenza across avian and human species, was co-expressed with coiled-coil oligomerization domains and assembled into SAPN in its native conformation as tetrameric state.23 The resultant SAPN was demonstrated that it elicited antigen specific antibodies and was able to reduce virus shedding in vaccinated chickens against low pathogenicity avian influenza virus (LPAIV).23 By adding SAPN a helix C epitope, a conserved stalk region of hemagglutinin, and a known molecule of flagellin domains as an immune stimulatory adjuvant, the resultant SAPN demonstrated that chickens vaccinated with self-adjuvanted SAPN containing M2e and helix C elicited high titer of cross-neutralizing antibodies against heterologous subtypes of AI viruses, make it a potential universal vaccine against AI.13 We also showed promising results of the SAPN vaccine against another model emerging disease, infectious bronchitis (our unpublished data). They demonstrated that chickens immunized with SAPN presenting spike epitopes induced high titer of antibodies, induced strong cellular immunity and protected chickens from challenge with a pathogenic strain M41(our unpublished data). Collectively, these promising results highlight SAPN could be a potential alternative to conventional vaccines for veterinary usage, especially for emerging viral diseases.
SAPN is a highly versatile vaccine platform. It has been demonstrated that SAPN as vaccine delivery system provides outstanding safety versus enhancing immunogenicity of the vaccines. Highlighted the promising progress in its application of designing vaccines for AI and IB pave the way for the next generation vaccines for many more veterinary diseases.
None.
None.

©2018 Li, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.