Journal of
eISSN: 2377-4282


Research Article Volume 5 Issue 4
1Department of Chemical Engineering, Brigham Young University, USA
2Department of Chemical Engineering, American University of Sharjah, UAE
Correspondence: William G Pitt, Department of Chemical Engineering, Brigham Young University, 350 Clyde Building, Provo, UT 84602, UAE, Tel 801-422-2589, Fax 801-422-0151
Received: April 14, 2017 | Published: May 8, 2017
Citation: Williams JB, Buchanan CM, Husseini G, Pitt WG (2017) Cytosolic Delivery of Doxorubicin from Liposomes to Multidrug-Resistant Cancer Cells via Vaporization of Perfluorocarbon Droplets. J Nanomed Res 5(4): 00122. DOI: 10.15406/jnmr.2017.05.00122
A common mechanism of multidrug resistance is the upregulation of efflux pumps in the cancer cells that can more rapidly export unwanted materials (e.g. cancer drugs) out of the cell, compared to sensitive cancer cells. This research seeks to overcome this mechanism by vaporizing a perfluoropentane emulsion droplet inside of a drug-containing liposome (eLiposome) that was endocytosed into a cancer cell. Folate attached to the eLiposome facilitates uptake into the cell as observed by confocal microscopy. Ultrasound was examined as a trigger to initiate the vaporization of the perfluoropentane droplet and release doxorubicin from folated eLiposomes (feLD). Two seconds of ultrasound released 78% of encapsulated doxorubicin from feLD. Doxorubicin-sensitive KB-3-1 cells and doxorubicin-resistant KB-V1 cells treated with feLD (without ultrasound) had cell viabilities of 33% and 60%, respectively. Ultrasound had negligible additional effect on the cell viability of KB-3-1 and KB-V1 cells treated with feLD (33% and 53%, respectively). We hypothesized that the doxorubicin sulfate fibers that were formed during the loading of doxorubicin into the eLiposome present a site for heterogeneous nucleation once the feLD is endocytosed by the cell, and thus droplet vaporization occurs with or without ultrasound.
Keywords:Controlled drug delivery, Doxorubicin, KB cancer cells, Perfluorocarbon droplet vaporization, Liposome, Multidrug resistance
Cancer cells that survive exposure to chemotherapeutics can often develop an acquired resistance to the administered drugs as well as other chemotherapeutic agents. This undesired phenomenon is known as multidrug resistance (MDR). One of the established mechanisms for multidrug resistance is the production of an increased number of export pumps,1-4 which increases the rate at which undesired compounds inside of the cell (such as cancer drugs) are pumped out of the cell, thus keeping internal concentrations below the therapeutic level even when conventional drug delivery provides therapeutic concentrations external to the cell. We posit that direct delivery to the cell cytosol can produce toxic internal concentrations without requiring excessive whole-body concentrations, even in the face of efflux pumps in MDR cells.
Drug delivery vehicles, such as liposomes, are often used to increase the concentration of drugs at the tumor site, while concurrently decreasing the concentration of free drugs in circulation and around healthy tissues.5 Gabizon et al.6 reported that PEGylated liposomal doxorubicin (LipoDox) released < 2% of encapsulated doxorubicin while in circulation. Carefully designed drug-containing liposomes can accumulate in cancerous tissues, along with their toxic payload. However, without a mechanism for quick release of the drug, killing of cancer cells still depends on the slow leakage of drugs from the liposomes and the subsequent diffusion of the free drug across the cell membrane. For MDR cells, this form of delivery is often too slow to be effective when using doses of drugs that are also non-lethal to the patient. On the other hand, a rapid drug release from the carrier will transiently spike the local drug concentration and transiently increase the internal concentration in the adjacent cells, hopefully high enough to produce toxicity. Even better is a scenario in which the drug-containing liposomes are endocytosed before the drug is quickly released; and in this scenario the release occurs inside the cell.
In our research reported herein, we combine internal delivery to the cytosol with rapid release in an effort to treat MDR cells. Ligands that induce receptor mediated endocytosis can promote uptake of the drugs into the cell, but the drugs must still be released quickly to escape the degradative environment of the endosomal pathway and spread to the cytosol and to the eventual site of action.
In this study, rapid release of a chemotherapeutic agent from liposomes is produced by the vaporization of a perfluorocarbon emulsion droplet loaded inside of a drug-containing liposome. This construct is called an eLiposome. Previous work has shown the successful synthesis of eLiposomes7 and the controlled delivery of calcein (a model drug)8,9 to the cytosol of non-MDR HeLa cells. Calcein was only observed in the cell cytosol when ultrasound was applied to folated eLiposomes loaded with calcein;8 thus ultrasound was used as a trigger for controlled release from eLiposomes. This process is called acoustic droplet vaporization. We hypothesize that delivery of a significant amount of drugs directly to the cytosol of a MDR cancer cell will increase both the concentration and the residence time of the drugs in the cell cytosol, and consequently increase the observed cytotoxic response. Therefore, the aim of this work was to determine if cytosolic delivery of doxorubicin (Dox) from folated eLiposomes, triggered by acoustic droplet vaporization, would enhance the killing of MDR cells.
Materials
The phospholipids 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-dipalmitoyl-sn-glycero-3-phosphate, sodium salt (DPPA) were purchased from Echelon Biosciences, Inc. (Salt Lake City, UT). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000], ammonium salt (DSPE-PEG-NH2) was obtained from Laysan Bio, Inc. (Arab, AL). Dodecafluoro-n-pentane (PFC5) was purchased from SynQuest Laboratories (Alachua, FL). Phosphate buffered saline, 10x solution (PBS), sodium chloride (NaCl), sodium hydroxide (NaOH), pyridine, ammonium sulfate ((NH4)2SO4), sodium lauryl sulfate (SDS), and Whatman® Nuclepore Track-Etch Membrane filters (19 mm diameter) were purchased from Fisher Scientific (Hampton, NH). Dulbecco’s modified eagle medium (1X) (DMEM) (+ 4.5 g/L D-glucose, + L-glutamine, - sodium pyruvate), RPMI 1640 (1X) (+ L-glutamine, + phenol red, - folic acid), penicillin streptomycin (Pen Strep), and fetal bovine serum (FBS) were purchased from Gibco® by Life Technologies (Grand Island, NY). 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Molecular Probes™ by Life Technologies (Eugene, OR). Trypsin-EDTA was purchased from Invitrogen (Carlsbad, CA). Sucrose, chloroform, and sulfuric acid (H2SO4) were purchased from Avantor Performance Materials, Inc. (Phillipsburg, NJ). Glycerol was purchased from MilliporeSigma (Billerica, MA). Nitrogen gas was purchased from Airgas (Salt Lake City, UT). Doxorubicin HCl injection, USP (10 mg/mL) was purchased from Pfizer (New York, NY). Vinblastine sulfate injection (1 mg/mL) was purchased from APP Pharmaceuticals, LLC (Schaumburg, IL). Cholesterol (powder, BioReagent, suitable for cell culture, ≥99.0%), folic acid (≥ 97%), ninhydrin, dimethyl sulfoxide, ≥99.9% (DMSO), methanol, N,Nˊ-dicyclohexylcarbodiimide, 99% (DCC), and L-glutamic acid potassium salt monohydrate (≥99%, (HPLC), powder) were purchased from Sigma-Aldrich (St. Louis, MO). Hydrochloric acid was purchased from Spectrum Chemical Mfg. Corp. (New Brunswick, NJ). Dimethyl sulfoxide-D6 (D, 99.9%) +0.05% V/V TMS DLM-10TB-10 was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). Poly-L-lysine (MW 30-70kD) 12 mm round, No. 1 German glass coverslips were purchased from Corning Inc. – Life Sciences (Oneonta, NY). Sephadex G-25 columns were purchased from GE Healthcare Life Sciences (Pittsburgh, PA). KB-3-1 and KB-V1 cells were kind gifts from Dr. Michael Gottesman (NIH, Bethesda, MA). Greiner Bio-One CELLSTAR® 24-well cell culture plates were purchased from BioExpress (Kaysville, UT). Cell culture flasks were purchased from Sarstedt AG & Co (Nümbrecht, Germany). All water used was deionized water distilled through a Corning Mega-Pure™ MP-1 Glass Still (ddH2O).
Folate was conjugated to DSPE-PEG-NH2 using a previously described method10 with slight modifications. Briefly, 16.7 mg of folic acid was dissolved in 0.667 mL of anhydrous DMSO. DSPE-PEG-NH2 was dissolved in 0.333 mL of pyridine and added to the folic acid solution. N,Nˊ-dicyclohexylcarbodiimide (DCC) (21.7 mg) was then added to the reaction mixture. Nitrogen gas was added to the round bottom flask and the round bottom flask was capped. The reaction proceeded under mild stirring for 4 hours at room temperature (~23°C) in the dark. Every hour the reaction progress was checked using thin layer chromatography on silica gel plates using a 75:36:6 chloroform/methanol/water mobile phase. Ninhidryin spray (0.2 g ninhydrin/100 mL ethanol) was used to confirm the disappearance of the amine on the DSPE-PEG-NH2. The pyridine was then removed by rotary evaporation and 6 mL of ddH2O was added to the mixture. The solution was centrifuged to remove trace insolubles and the supernatant was dialyzed in 3500 molecular weight Spectra/Por® (Spectrum Laboratories, Inc., Rancho Dominguez, CA) tubing against NaCl (50 mM, 2 x 700 mL) and then against ddH2O (3 x 700 mL). An equal amount of chloroform was added to the dialysate to extract the product (DSPE-PEG-folate). Hydrochloric acid was added to the aqueous phase to make the product more soluble in chloroform, as was seen by the shift of the characteristic yellow of folate from the aqueous to chloroform phase. This chloroform phase was collected and stored at 4°C. H-NMR confirmed the synthesis of DSPE-PEG-folate (in DMSO-d6).
eLiposome synthesis
eLiposomes were synthesized using a modified procedure of Javadi et al.7 designed to make larger sized eLiposomes. Briefly, DPPA in chloroform was dried on a flask using rotary evaporation and was subsequently rehydrated (5 mg/mL) in 129 mM ammonium sulfate (pH 4.5). Once cooled, PFC5 (0.02g/mg DPPA) was added to the DPPA solution. The mixture was then sonicated on ice with a 20-kHz probe (Sonics and Materials, CVX400, Newton, CT) at 1.25 W/cm2 (30% amplitude setting) for five 1-min intervals with a 1 min pause between each sonication. The DPPA-coated PFC5 emulsion droplets were extruded (LiposoFast™, Avestin, Ottowa, ON, Canada) through a 100-nm polycarbonate track-etched filter (Whatman® Nuclepore).
Liposomes were synthesized by drying 37.5 mg DPPC and 12.5 mg cholesterol onto a glass round bottom flask. For folated liposomes, 0.2 mL of the DSPE-PEG-folate in chloroform (see section 2.2) was added to the lipids before drying. The lipids were then rehydrated (50 mg/mL) in 129 mM ammonium sulfate (pH 4.5) and extruded through a 400-nm polycarbonate filter to make a uniform distribution of unilamellar liposomes.
The eLiposomes were made by mixing 1 mL of DPPA-coated PFC5 emulsion droplets and 1 mL of liposomes. This solution was sonicated (1 W/cm2) on ice for three 15-s intervals with 60-s pauses between each sonication. eLiposomes are separated from external emulsion droplets and empty liposomes using a pillow density separation technique. Briefly, eLiposomes followed by NaCl and then sucrose (~0.4 mL each) were carefully pipetted into the bottom of a microcentrifuge tube and centrifuged at 3000 rpm for 10 min. The eLiposomes accumulated between the NaCl and sucrose layers and were collected and passed through a Sephadex G25 column to replace the external media with PBS (pH 7.4), establishing a transmembrane pH gradient.
Doxorubicin was loaded into eLiposomes using a transmembrane pH gradient. An equal volume of Dox in PBS (0.1 mg/mL) was added to the eLiposomes and left for 18 hrs at 4°C. The solution was then pipetted and centrifuged for 10 min at 3000 rpm (735 x g). The Dox-loaded eLiposomes (eLipoDox) were resuspended in PBS and diluted to an absorbances value of 0.5 (Beckman Coulter DU-640 UV, Fullerton, CA) measured at 480 nm. This corresponds to a Dox concentration of 0.024 mg/mL, or about 41 µM.
Folated eLipoDox and non-targeted eLipoDox were sized using dynamic light scattering (Nano Brook Omni, Brookhaven Instruments Corporation, Holtsville, NY). The zeta potential of folated Liposomes and non-targeted liposomes were measured using phase analysis light scattering (Nano Brook Omni, Brookhaven Instruments Corporation, Holtsville, NY).
Doxorubicin release experiments
Experiments investigating the release of doxorubicin from eLiposomes using ultrasound (Sonics and Materials, CVX400, Newton, CT) were conducted using a QuantaMaster fluorometer (Photon Technology International, Birmingham, NJ, USA). Folated eLipoDox or non-targeted eLipoDox (20 µL) was added to PBS (2 mL) in a cuvette and gently mixed by re-pipetting. Fluorescence was measured using excitation and emission wavelengths of 475 nm and 588 nm, respectively. The cuvette was removed and ultrasound was applied (1 W/cm2, 20 kHz, 2 s total of US applied in 20 sec of 0.1-s pulses, 1:10 duty cycle). The cuvette was placed in the spectrometer and the fluorescence measured again. This procedure and measurement were repeated one more time to produce a second round of release. Then SDS (30 µL) was added to the solution and gently re-pipetted to release all of the Dox, and the fluorescence was then measured.11 The % Release was caclucated using the following equation
(1)
Where is the initial (baseline) fluorescence, is the fluorescence after sonication, and is the fluorescence after Dox release using SDS.
Cell culture
KB-V1 (Dox resistant) cells' and their parent cell line, KB-3-1 (Dox sensitive), were kind gifts from Dr. Michael Gottesman (NIH, Bethesda, MD). The KB-V1 cells have an acquired multi-drug resistance produced from culturing KB-3-1 cells in increasing concentrations of vinblastine.12 KB-3-1 and KB-V1 cells were cultured in DMEM containing 10% FBS and 1% Strep Pen and incubated at 37°C, 5% CO2. Vinblastine (3.5 µg/5 mL DMEM) was added to the KB-V1 cells growth media to maintain its multidrug resistance. Before experiments, cells were washed and grown for 48 hours in folate-free RPMI media (10% FBS, 1% Step Pen), and no vinblastine was added to KB-V1 cells. Cells were seeded in a 24-well plate 24 hours before the addition of drugs at an approximate cell density of 1 x 104 and 2 x 104 for KB-3-1 and KB-V1 cells, respectively.
Confocal experiments
KB-V1 cells were seeded at approximately 6 x 104 cells on poly-L-lysine (MW 30-70kD) 12 mm round, No. 1 German glass coverslips in wells of a 24-well plate. Cells grew for 48 hours in RPMI folate-free media before the addition of folated or non-folated eLipoDox (0.2 mL, 0.5 Abs at 480 nm). Drugs were allowed to incubate (37°C, 5% CO2) for 2 hours before the media was removed. Cells were then rinsed with 0.5 mL of ice-cold PBS. The PBS was removed and 1.0 mL of ice-cold methanol was added to the cells and the 24-well plate was kept on ice for 10 minutes. The methanol was removed and the cells were rinsed twice with ice-cold PBS. The cell-covered cover slips were then placed on plain selected precleaned VWR micro slides (25 x 75 mm, 1.0 mm thick, VWR International, LLC, Radnor, PA) using 5 µL of a 50% glycerol/ddH2O solution. Confocal images were obtained using an Olympus FluoView FV1000 (Tokyo, Japan) confocal microscope.
MTT assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was used to measure the metabolic activity of living cells.13 For cell experiments, 0.1 mL of MTT in PBS (5 mg/mL) was added to the cells (0.9 mL growth media) and incubated at 37°C, 5% CO2 for 2 hours. The growth media containing MTT was removed and the formazan was solubilized using 1.0 mL of DMSO. After 15 min, the absorbance at 570 nm and 700 nm were measured using a Synergy™ MX multimode microplate reader (BioTek® Instruments, Inc., Winooski, VT). Seeding density for cells was optimized to obtain an absorbance value (570 nm) of approximately 1.0 for the PBS control.
IC50 experiments
Doxorubicin (0.2 mL) was added to the cells (1.0 mL growth media) in a 24-well plate to obtain Dox concentrations between 0.001 and 500 µM. PBS was used as a positive control. The media was removed after 2 hours of incubation (37°C, 5% CO2) and the cells were rinsed with 0.5 mL of PBS. The PBS was then replaced with fresh DMEM (10% FBS, 1% Pen Strep). The MTT assay was performed 48 hours later.
Ultrasound experiments with folated eLipoDox
After KB-3-1 and KB-V1 cells were seeded and grown in a 24-well plate, 0.2 mL of PBS, Dox, eLipoDox, or folated eLipoDox was added to the 1.0 mL of RPMI in the wells and gently mixed. Before addition, the Dox, eLipoDox, and folated eLipoDox suspensions were adjusted to have an absorbance of 0.5 measured at 480 nm (Beckman Coulter DU-640 UV, Fullerton, CA), which corresponds to a final diluted Dox concentration of 7 µM in the wells. Cells incubated (37°C, 5% CO2) for 2 hours with the drugs. To minimize the cellular damage or detachment of the cells from the bottom of the well during insonation, 1.8 mL of DMEM was added to all of the cells just before ultrasound was applied to some of the cells (1 W/cm2, 20 kHz, 2 s total of US applied in 20 sec of 0.1-s pulses, 1:10 duty cycle). The ultrasonic probe was placed in the growth media so that the tip was just below the liquid surface. Following ultrasound, the media was removed and the cells were rinsed with 0.5 mL PBS to remove any drug not internalized by the cells. After removing the PBS, 0.9 mL of fresh DMEM (10% FBS, 1% Pen Strep) was added to the cells. An MTT assay was performed 48 hours after insonation.
Dox loading with glutamate vs sulfate
Folated eLiposomes were synthesized as described in section 2.3 using either ammonium sulfate or potassium glutamate to rehydrate the liposomes and emulsion droplets. In a limited number of experiments, doxorubicin was loaded into folated eLiposomes using a pH gradient as described in 2.4, except that the Dox solution added to folated eLiposomes had a concentration of up to of 2 mg/mL, instead of 0.1 mg/mL. In some experiments the loading process was at 4°C for 17 hours, and then the folated eLipoDox solutions remained at room temperature (23°C) for an additional 90 min.
Viability of KB cells
Various concentrations of free (soluble) Dox were administered to both KB-V1 and KB-3-1 cells to confirm the resistance of the KB-V1 cells to Dox and to determine an optimal concentration for subsequent experiments with folated eLipoDox. Cell viability was determined via an MTT assay.
Figure 1 shows that the dose response curve for the MDR KB-V1 cells is shifted to the right (higher Dox concentration) compared to KB-3-1 cells, indicating that the KB-V1 cell line is indeed more resistant to treatment with free Dox compared to the KB-3-1 cell line. The shift is about 2 log units, indicating that 100-fold more Dox (external to the cell) is required to produce a similar toxicity in the MDR KB-V1 cell line even though this cell line had been developed by vinblastine exposure, proving its multidrug-resistant behavior. The Dox IC50 values of KB-3-1 and KB-V1 cells were determined using a four parameter logistic equation, and the four parameters were optimized using a least-square regression analysis. KB-3-1 and KB-V1 cells have a Dox IC50 of 1.3 µM and 184.0 µM, respectively, which shows that the KB-V1 cells are 147 times more resistant to a 2-hour treatment of free Dox than are KB-3-1 cells. As seen in Figure 1, there is a sharp decrease in cell viability for KB-3-1 cells between 1 (~59% viability) and 10 µM (~9%), whereas there is no statistical difference for the KB-V1 cells between 1 (~81%) and 10 µM (~86%). Therefore, we used a Dox concentration of 7 µM for further experiments to provide a high sensitivity to examine if folated eLipoDox can reduce the viability of resistant KB-V1 cells to match that of sensitive KB-3-1 cells, thus effectively reversing (or overcoming) the multidrug resistance of KB-V1 cells.
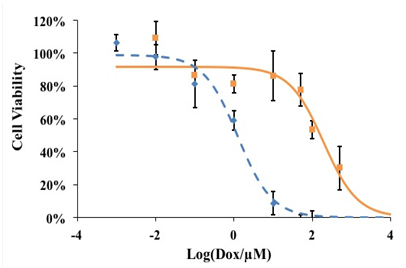
Figure 1 Dose response curve for KB-3-1 (♦) and KB-V1 cells (█) treated with Dox. Lines represent the fit to a four parameter logistic equation using a least square regression analysis. Error bars represent the standard deviation of experimental data points (n=3).
Characterization of folated eLipoDox
eLiposomes were synthesized using slightly modified published procedures7 as described in section 2.3 and were made with or without targeting folate ligands on the surface. The zeta potential of folate-targeted liposomes was -21.41 ± 1.85 mV (mean ± s.d.), whereas the zeta potential of non-targeted liposomes was -9.48 ± 0.60 mV. The more negative zeta potential of folated liposomes confirms the successful incorporation of DSPE-PEG-folate into the surface of the targeted liposomes, as at neutral pH, the folic acid groups are ionized and negatively charged. Synthesized eLipoDox had an average diameter of 448 ± 16 nm (mean ± 95% c.i.) as determined by dynamic light scattering, and was not statistically different (p=0.28) in size compared to folated eLipoDox, which had an average diameter of 469 ± 36 nm. The concentrations of Dox, LipoDox and eLipoDox suspensions were determined using UV/VIS spectrophotometry. The peak absorbance at 480 nm was used with an extinction coefficient of 12,000 M-1 cm-1 as determined via a calibration curve of free Dox. For these experiments the target absorbance at 480 nm was 0.50, which corresponds to a Dox concentration of 41 µM. When 0.2 mL of this was mixed with 1.0 mL of cell media, the concentration to which the cells were exposed was 7.0 µM.
Uptake of folated and non-folated eLipoDox
The first set of experiments with eLipoDox was designed to see if eLipoDox particles had been taken inside of KB-V1 cells by the time ultrasound would be applied. The liposomes were tagged with a fluorescent label (DiI), and folate-targeted (Figure 2A & 2C) and non-targeted (without folate, Figure 2B & 2D) eLipoDox were delivered to KB-V1 cells. After 2 hours, the cells were rinsed with PBS and imaged using a confocal microscope (no US was applied). Any fluorescence appearing in Figure 2C& 2D is the result of DiI in the liposome membranes, which liposomes are either attached to the surface of the cell or inside of the cell. A sample slice from the stack of confocal images taken is shown in Figure 2A & 2D, corresponding to the slice with the highest fluorescence. For the cells exposed to folated eLipoDox, every cell appears to emit fluorescence (Figure 2C); whereas when the cells were exposed to eLipoDox without a targeting ligand, little to no fluorescence is observed (Figure 2D). Furthermore the confocal slices indicated that fluorescence appears to be found in the cell cytosol, not solely at the cell surface. This suggests that folate promotes cytosolic uptake of liposomal doxorubicin to KB-V1 cells.
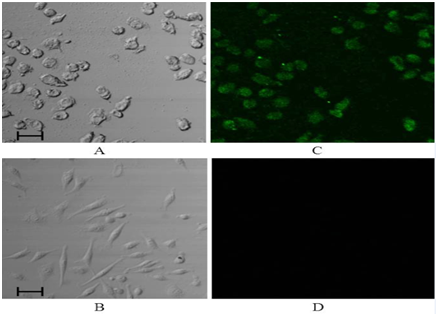
Figure 2 Light (A) and confocal (C) images of KB-V1 cells exposed to folated eLipoDox for 2 hours (no US). Light (B) and confocal (D) images of KB-V1 cells exposed to eLipoDox for 2 hours (no US). These constructs were labeled with DiI in their bilayer membrane, which gives rise to the fluorescence in the confocal images. The scale bar indicates 50 µm.
Dox release from eLipoDox
Previous studies8,9,14 showed that calcein (a model drug) could be released from eLiposomes upon insonation. Lattin et al.9 synthesized 800-nm liposomes with PFC emulsion droplets of two sizes (100 nm and 400 nm). They observed approximately 20% release of calcein from PFC5 eLiposomes after 0.1s of 20-kHz US (1 W/cm2), and the release increased with time (~ 78% release after 5s for 400 nm droplets, 40% release for 100 nm droplets). They also looked at smaller (200 nm) liposomes with 100 nm droplets and observed less release (~ 12% release after 1s and 21% after 5s, 20 kHz, 1 W/cm2) compared to the larger liposomes.14
A similar study was performed in order to determine the release of doxorubicin from eLiposomes. Dox is self-quenched at high concentrations;15 therefore, the fluorescence of Dox will increase as it is released from the liposome and diluted. The increase in the fluorescence of Dox is proportional to the amount released. A QuantaMaster fluorometer (Photon Technology International, Birmingham, NJ, USA) measured the fluorescence of Dox released from folated eLipoDox and folated LipoDox (no emulsion droplet) after insonation using excitation and emission wavelengths of 475 nm and 588 nm, respectively. The same ultrasound conditions applied to cells in viability experiments (section 3.5) were used in the release experiments (1 W/cm2, 20 kHz, 2 s total of US applied in 20 sec of 0.1-s pulses, 1:10 duty cycle).
Average Dox release was calculated using Equation 1 and the results are plotted in Figure 3. Folated eLipoDox released an average of 78% of encapsulated Dox after 2 s of pulsed US, whereas folated LipoDox only released 29% (Figure 3, p=0.0002). The presence of an emulsion droplet inside of the liposome caused a significant increase in the release of Dox from liposomes. The majority of Dox was released from folated eLipoDox after only 2 s of pulsed US. An additional 2 s of US did not produce any further significant increase in Dox released from folated eLipoDox compared to the first 2 s of US (82% total release compared to 78%, p=0.25); however, an additional 2 s of US did show significant increase in the release of Dox from folated LipoDox (no emulsion droplet) (29% to 51%, p=0.02).
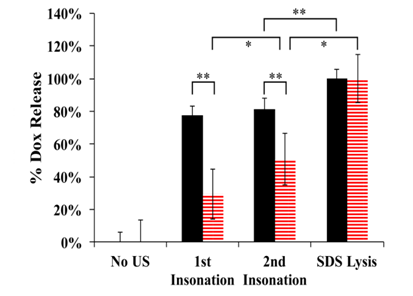
Figure 3 Average Dox release from folated eLipoDox (feLD, black/solid) and folated LipoDox (fLD, red/hatched) measured by fluorescence. A baseline fluorescence (No US) was first measured. US was then applied twice (1 W/cm2, 20 kHz, 2 s total of US applied in 20 sec of 0.1-s pulses, 1:10 duty cycle) with the fluorescence measured after each insonation. Finally, SDS was added to achieve 100% release. Error bars represent the 95% confidence interval. (n=3).
*indicates statistical difference at p < 0.05.
**indicates statistical difference at p < 0.01.
Previous data by Javadi et al.7 showed that the presence of both a targeting folate ligand on the surface of the eLiposome and an emulsion droplet inside the liposome increased the calcein fluorescence inside of HeLa cells after insonation.8 Our results show that folated eLipoDox is taken into the cells after 2 hours (Figure 2C), and that a 2-sec pulse of US can cause the release of the bulk of encapsulated Dox from folated eLipoDox (Figure 3). These results suggest that US can be used to trigger an instantaneous release of Dox from folated eLipoDox directly to the cell cytosol of MDR cells.
We investigated the viability of KB cells after the delivery of folated eLipoDox and the application of US. Folate-targeted and non-targeted eLipoDox, as well as folate-targeted LipoDox (no emulsion droplet) were synthesized and diluted to approximately 7 µM in the cell suspension. Both KB-V1 and KB-3-1 cells were incubated with either PBS, free Dox (7 µM), folated eLiposomes (No Dox), folated LipoDox (no emulsion droplet), eLipoDox, or folated eLipoDox for 2 hours before the samples were insonated in selected wells in a 24-well plate (1 W/cm2, 20 kHz, 2 s total of US applied in 20 sec of 0.1-s pulses, 1:10 duty cycle). After US was applied to the selected wells, all of the cells were rinsed with 0.5 mL of PBS. The PBS was removed and 0.9 mL of fresh growth media was added to the wells. An MTT cell viability assay was performed 48 hours after insonation and the cell viability results are shown in Figure 4. The percent viability for each condition is referenced to cells exposed to PBS but without any insonation (PBS No US) for the given cell line (resistant or sensitive). At the concentration of Dox used in these experiments (7 µM), the viability of MDR KB-V1 cells exposed to free Dox is above 90% with or without ultrasound, suggesting that the resistant cancer cells can quickly export any Dox that diffuses into the cell. KB-3-1 cells are unable to export Dox as quickly due to the lack of P-gp pumps, compared to KB-V1 cells,16 as can be seen from the lower viability (~47%) in response to treatment with free Dox.
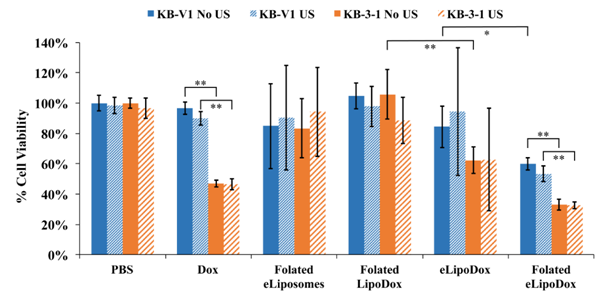
Figure 4 Mean cell viability of KB-V1 (blue) and KB-3-1 (orange) cells measured by an MTT assay 48 hours after drug delivery. Concentration of Dox is approximately 7 µM. Cells were either treated with US (hatched bars, 1 W/cm2, 20 kHz, 2 s total of US applied in 20 sec of 0.1-s pulses, 1:10 duty cycle) or without US (solid bars). Error bars represent the 95% confidence interval. (n ≥ 3).
*indicates statistical difference at p < 0.05.
**indicates statistical difference at p < 0.01.
Cytotoxicity of folated eLiposomes
KB-V1 and KB-3-1 cells were both treated with folated eLiposomes without Dox to determine the cytotoxicity of our drug delivery vehicle. During the synthesis of folated eLipoDox, the folated eLiposomes were split into two separate vials before loading Dox into the eLiposomes. Dox in PBS was added to one vial (folated eLipoDox), and to the other vial an equal amount of PBS was added (folated eLiposomes without Dox). Folated eLiposomes were then treated the same way as the folated eLipoDox. KB-V1 cells had a cell viability of 85% and KB-3-1 cells had a cell viability of 83% when treated with folated eLiposomes (no Dox) without US (not statistically different). Cell viability slightly increased for both KB-V1 cells (90%) and KB-3-1 cells (94%) when US was applied, but the change was not statistically significant. The viability of cells exposed to folated eLiposomes without Dox averaged less than 100%, but was not statistically different than any formulation without Dox.
Other studies17,18 investigating the cytotoxicity of different cell lines treated with Dox liposomes attached to PFC microbubbles similarly report 10-20% killing of cells treated with just their constructs and US (no Dox). In this study, the killing of KB cells treated with folated eLiposomes with or without US is not statistically significant compared to the PBS only control (Figure 4). Thus, folated eLiposomes without drug have no statistically significant effect on the cell viability of KB cells.
Effect of ultrasound
Some cells were treated with PBS and US to verify that the acoustic conditions did not have a significant effect on the cell growth. The results show that there is no significant difference due to the application of only US to KB-V1 (99%) or KB-3-1 (97%) cells (p>0.05 in both cases). Ultrasound did significantly increase the killing of KB-V1 cells treated with free Dox, decreasing the viability from 97% to 90% (p=0.029). This slight decrease in viability could be attributed to the cell membrane becoming more permeable for a short time after insonation, which is not unusual,19 thus increasing the influx of Dox. The cells were washed within 20 minutes of US being applied, which would allow a short time for a small amount of free Dox to more easily diffuse into the cell than normally would enter without insonation.
Figure 3 show that ultrasonic insonation can release approximately 29% of encapsulated Dox from folated LipoDox (no emulsion droplet) and 78% from folated eLipoDox. Although insonation produces 29% release of Dox from folated LipoDox in the absence of cells, there is no significant difference in viability due to insonation when folated LipoDox was used against KB-V1 or KB-3-1 cells. There is, however, a significant (p=0.047) decrease in the cell viability observed after insonation of KB-V1 cells treated with folated eLipoDox. Yet, while statistically significant, the decrease in viability is not substantial (from 60% to 53%). The reason for this relatively small decrease in viability is attributed to the instability of the folated eLipoDox once it has been endocytosed by the cell, and therefore produces lower viability of cells exposed to folated eLipoDox even when not insonated (60% for KB-V1 cells, 33% for KB-3-1 cells). Surprisingly, ultrasound has a negligible effect on the viability of KB cells for any of the conditions shown in Figure 4, which includes folated eLiposomes with and without Dox in the liposomes. While ultrasound does not produce a pronounced difference, Dox produces a significant difference, as does folate, which will be discussed next.
Effect of folate
As discussed previously, folate induces much more uptake of eLipoDox. Figure 2 shows the uptake of folated eLipoDox compared to non-folated eLipoDox. The cells shown in Figure 2 were rinsed with PBS prior to preparation for confocal microscopy, so only free Dox inside of the cell or eLipoDox inside of or bound to the cell was present to create the fluorescence in the image. This result suggests that folated eLipoDox can release a significant amount of Dox to the cytosol of the cell, whereas eLipoDox does not induce uptake by the cell, so there is a negligible amount of Dox released directly to the cell cytosol. This is supported by the cell viability data shown in Figure 5. KB-V1 cells have a viability of 84% when treated with eLipoDox (Figure 5A), which is statistically higher when compared to cells treated with folated eLipoDox (60%, p=0.003), but not statistically different when compared to cells treated with free Dox (97%, p=0.076).
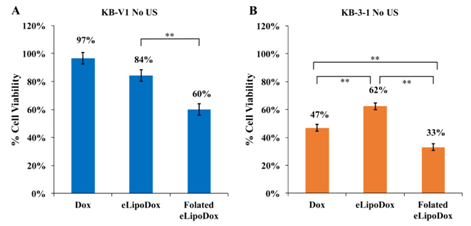
Figure 5 Cell viability of resistant KB-V1 (A) and sensitive KB-3-1 (B) cells treated with Dox, eLipoDox, or folated eLipoDox. No Ultrasound was applied. Error bars represent 95% confidence interval (n≥8).
*indicates statistical difference at p < 0.05.
**indicates statistical difference at p < 0.01.
The importance of a folate ligand for cytosolic delivery of Dox is also observed by comparing the viability of KB-3-1 (sensitive) cells treated with Dox, eLipoDox or folated eLipoDox (Figure 5B). Non-folated eLipoDox kills significantly less KB-3-1 cells (62%) than either free Dox (47%, p<0.01) or folated eLipoDox (33%, p<0.0001). It is unlikely that large amounts of eLipoDox (without folate) are taken up by the cell during the 2 hours of incubation (Figure 2D). Gabizon et al. report that PEGylated liposomal doxorubicin (no emulsion droplet) passively accumulates in the tumor interstitial fluid and gradually releases doxorubicin without any significant interaction with tumor cells.20 Thus, Dox released from eLipoDox (either by diffusional escape or by PFC5 droplet vaporization) will remain outside of the cell, and the concentration of free Dox outside of the cell will be less than that of cells treated with free Dox. Since in our experiments the media containing any drug is removed after 2 hours of incubation with eLipoDox, and the cells are rinsed with PBS to reduce any residual drug, there is minimal time for any Dox released from eLipoDox to diffuse into the cell. Attaching folate to eLipoDox facilitates endocytosis by the cell (Figure 2C); thus, Dox can be released directly from folated eLipoDox to the cell cytosol. Cell viability of KB-3-1 cells treated with folated eLipoDox (33%, Figure 5B) is significantly less than the cell viability of Dox-treated KB-3-1 cells (47%, p<0.0001). These results support the hypothesis that folate greatly enhances the cytosolic delivery of doxorubicin from eLipoDox.
Effect of emulsion droplet
As mentioned, KB cells were treated with folated LipoDox (no emulsion droplet) to confirm that an emulsion droplet is necessary to get adequate release to the cytosol of the cells. There is little to no killing of KB-V1 or KB-3-1 cells treated with folated LipoDox (Figure 6); however, cell viability is significantly reduced (p<0.0001, No US) for both KB-V1 and KB-3-1 cells when there is an emulsion droplet encapsulated inside the liposome (folated eLipoDox). The presence of an emulsion droplet makes a statistically significant difference, with or without ultrasound.
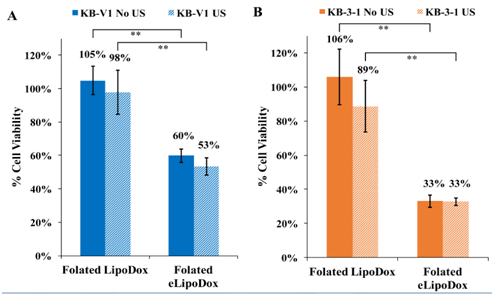
Figure 6 Cell viability of resistant KB-V1 (A) and sensitive KB-3-1 (B) cells treated with folated LipoDox or folated eLipoDox. Error bars represent 95% confidence interval (n≥3).
*indicates statistical difference at p < 0.05.
**indicates statistical difference at p < 0.01.
The presence of an emulsion droplet inside of folated eLipoDox apparently provides a possible mechanism for the release of Dox from the liposome directly to the cell cytosol. The PFC5 droplet (100-200 nm) will expand 5 times in diameter (500-1000 nm) when it vaporizes, thus rupturing the liposome and most likely disrupting the endosome as well, as endosomes are less than 1 µm in size.21 This will provide a greater opportunity for Dox molecules to reach the nucleus and initiate apoptosis. Interestingly, ultrasound does not appear to be necessary to provide a significant amount of killing of KB-V1 or KB-3-1 cells with folated eLipoDox, and insonation provides only a marginal increase in the killing of KB-V1 cells (Figure 6).
Instability of endocytosed folated eLipoDox
As mentioned earlier, one possibility for the significant amount of killing when cells were incubated with folated eLipoDox (in the absence of US) is that the folated eLipoDox is not stable once it is endocytosed by the cell. In order for US to trigger the release of doxorubicin from eLipoDox to deliver it to the cell cytosol, the eLipoDox construct must be endocytosed and the PFC5 droplet must vaporize when, and only when, US is applied. We hypothesize that there is significant killing when KB cells are treated with folated eLipoDox (but without insonation) because, as the folated eLipoDox is endocytosed by the cell, the PFC5 droplet vaporizes, whether or not US is applied. This hypothesis is consistent with all the data presented in this article, but is difficult to prove.
Our proposed mechanisms underlying this hypothesis is that the Dox precipitates with sulfate to form fibrous crystals during the process of loading doxorubicin. These solid fibers provide nucleation sites for the PFC5 molecules inside of the liposome to nucleate to a gas bubble, independent of the application of US. When folated eLipoDox enters the cell through folate-mediated endocytosis, the PFC gas bubble forms spontaneously, releasing Dox to the cell cytosol without application of ultrasound. This hypothesis was explored and tested by gleaning results from literature and from additional quantitative and qualitative experiments.
The formation of fibrous crystals of Dox sulfate when using an ammonium sulfate pH gradient has been reported by many groups.22 But there are other reports that Dox fibers form when liposomes are loaded using a citrate pH gradient23,24 and a magnesium sulfate pH gradient.25 These studies show that the precipitated Dox salts will form bundles of fibers as the drug to lipid (D/L) mass ratio increases, with simple rod-like fibers forming at least by a D/L ratio of 0.05. As the D/L ratio increases further, the fibers become thicker and appear to take up more volume inside the liposomes, with some shapes of the Dox crystals appearing to be circular or triangular and even globular at higher (D/L) ratios.23,25 The Dox fibers can even distort the spherical shape of the liposomes, which would introduce significant stress on the liposomal membrane.
Both reports noted that the percentage of encapsulated Dox eventually released from liposomes decreased as the D/L ratio increased.23,25 Additionally, Li et al.23 showed that the significant increase in the retention of Dox inside of the liposomes corresponds to the Dox fibers forming bundles of fibers.23 We postulate that these Dox fibers provide heterogeneous nucleation sites for the initiation of the PFC5 gas phase, and that the liquid droplet will provide PFC5 molecules to form a gas phase, whether or not US is applied. For our experiments, the initial D/L ratio is 0.005, assuming no lipids are lost during the synthesis procedure. It should be noted that some lipids are lost during the pillow density separation (process that separates empty liposomes from eLiposomes and emulsion droplets), although the exact amount of loss has not been quantified.
To test this nucleation postulate, experiments were performed with folated eLipoDox to determine the effect of the initial drug to lipid ratio. Folated eLiposomes were synthesized and split into multiple vials, with each vial having the same lipid concentration. Various concentrations of Dox in PBS, but equal liquid volumes, were then added to the multiple vials. At the highest concentration of Dox (2 mg/mL) used in these experiments, the initial D/L ratio is 0.16, assuming no lipids are lost. But even in the most dubious case of losing 90% of lipids, the ratio would be even higher, sufficiently high to form fibrous crystals.
We also expect that because there is a PFC5 emulsion droplet in the liposome, there will be a maximum amount of Dox loading before the emulsion droplet would be crowded into touching the Dox fibers and forming vapor, even before delivering eLipoDox to the cells. Thus, eLipoDox loaded at very high D/L ratios are expected to be the most unstable and therefore the most likely for the PFC5 emulsion droplet to vaporize inside of the liposome (due to less space inside of the liposome for the emulsion droplet). We expect eLipoDox at lower D/L ratios to be more stable and have higher cell viability when not insonated. When the D/L ratio is too low, few if any Dox crystals would form and molecular Dox would be more likely to permeate out23 before the folated eLipoDox could be endocytosed; thus we would expect the cell viability to also be higher (especially for KB-V1 cells) because the Dox would escape before endocytosis, and Dox would need to diffuse across the cell membrane into the cell to reduce cell viability, which is less likely at these lower Dox loadings.
We performed several experiments with Dox loading concentrations between 0.05-2 mg/mL, corresponding to D/L ratios between 0.004 and 0.16 (assuming no lipids are lost during synthesis). The D/L ratios would increase if lipids were lost during synthesis (i.e. if 50% lipids are lost, then D/L ratios are between 0.006 and 0.32). We were not able to make and measure a D/L ratio lower than 0.001 due to limitations in detector sensitivity.
Figure 7 shows that except for the peak at the Dox loading of 0.25 mg/mL, there is no significant and meaningful difference due to the D/L ratio in cell viability of KB-3-1 or KB-V1 cells. Furthermore, there is no significant difference in viability between cells with and without insonation (data not shown) for the D/L ratios investigated. These results suggest that even a low concentration of Dox loading is sufficient to provide a possible nucleation site for PFC5 emulsions also loaded inside the liposome.
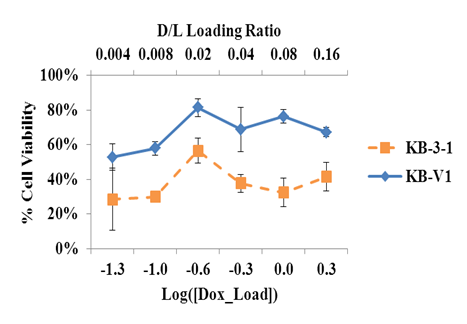
Figure 7 Cell viability of KB cells treated with folated eLipoDox with different initial drug to lipid ratios. The x-axis refers to the logarithm of the initial Dox concentration (mg/mL) added to an equal volume of folated eLiposomes. If no lipids are lost during the synthesis of folated eLiposomes, the largest D/L ratio would be 0.16. Error bars refer to the 95% confidence interval (n≥4).
Further support of the heterogeneous nucleation of gas was provided by doing experiments in which Dox fibers are not formed. Li et al.23 show that Dox fibers are not formed when Dox is loaded using a monoanionic buffer such as glutamate. We performed qualitative experiments comparing the generation of gas bubbles when Dox is loaded into folated Liposomes containing a PFC5 emulsion droplet using glutamate pH gradients versus ammonium sulfate pH gradients. Our hypothesis suggests that there would be the nucleation of bubbles when ammonium sulfate is used as the pH gradient due to the presence of a Dox fiber inside of the eLiposome. Minimal bubbles will be observed, if any, when glutamate is used because no Dox fibers are formed during the loading process and no Dox fibers would nucleate the PFC5 liquid droplets to gas.
In separate experiments, Dox was loaded into folated eLiposomes using an ammonium sulfate pH gradient or a potassium glutamate pH gradient. An equal volume of Dox in PBS (2 mg/mL) and folated eLiposomes were added together and allowed to incubate in the refrigerator (4°C) overnight for 17 hours. The next morning (at 17 hours) bubbles were observed in the vial containing folated eLipoDox formed using an ammonium sulfate pH gradient, but no bubbles were observed in the vial containing folated eLipoDox formed using a potassium glutamate pH gradient (Figure 8A). Then the vials were kept at room temperature (23°C) for 90 additional minutes (Figure 8B) after which we observed more (and larger) bubbles for the ammonium sulfate loaded eLipoDox sample. There were also a few small bubbles that did appear in the vial with folated eLipoDox loaded using a glutamate pH gradient. Loading of Dox in folated eLiposomes for experiments in section 3.5 used an ammonium sulfate pH gradient at 4°C and 20 hrs, and a Dox concentration of 0.1 mg/mL; yet no bubbles were observed. The observation of a significant amount of bubbles when loading 2 mg/mL Dox in the ammonium sulfate feLD (but not in the glutamate feLD, Figure 8) supports our postulate that the presence of a Dox fiber bundle provides a nucleation site to form perfluoropentane gas bubbles.
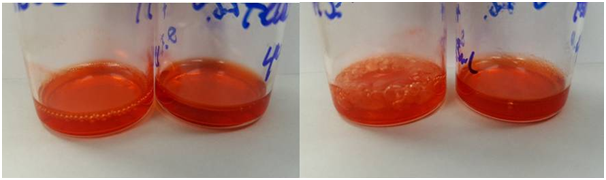
Figure 8 Folated eLipoDox after synthesis and incubation with Dox (2mg/mL). Right vial in (A) and (B) is Dox loading with potassium glutamate. Left vial in (A) and (B) is Dox loading with ammonium sulfate. (A) Immediately after loading Dox for 17 hours at 4°C. (B) After 90 additional minutes sitting at room temperature (23°C).
Li et al.23 report that when glutamate is used as the pH gradient, Dox could not be loaded to as high of a Dox concentration inside of the liposome because Dox does not form fibrous crystals.23 They also report that at the maximum loading of Dox inside glutamate liposomes, 60% of the Dox is released within 30 minutes in 50% human plasma. In comparison, Dox that formed fibrous bundles with citrate released less than 10% of Dox after 30 minutes in 50% human plasma. Thus while loading folated eLipoDox using a glutamate gradient is a possibility, the resulting construct would not be useful in a clinical application.
Dox-sensitive KB-3-1 cells and Dox-resistant KB-V1 cells were used to investigate the efficacy of cytosolic delivery of Dox in overcoming multidrug resistance. The folated eLiposomes (no Dox) used in this study had minimal effect on the growth of KB cells (10-20% killing) and viability was not statistically different from the negative control of PBS without insonation. Folated eLipoDox was shown to be taken into cells during 2 hours of incubation, whereas there was no observable uptake of non-folated (non-targeted) eLipoDox after 2 hours. Release of the drug inside the cell, as is the case with folated eLipoDox, significantly increases the killing of sensitive KB-3-1 (47% to 33%) cells and resistant KB-V1 (97% to 60%) cells compared to cells treated with free Dox. Non-targeted eLipoDox actually killed KB-3-1 cells less than free Dox (62% v 47%) presumably because the construct remains external to the cells when the Dox is released, and the lower concentration of external free Dox results in a lower driving force for Dox to diffuse into the cell.
Ultrasound at mild conditions (1 W/cm2, 20 kHz, 2 s total of US applied in 20 sec of 0.1-s pulses, 1:10 duty cycle) can release approximately 78% of Dox encapsulated in folated eLipoDox in vitro. Despite folated eLipoDox being endocytosed by cells and US being able to release Dox, there is no substantial difference in cell viability of KB-3-1 or KB-V1 cells due to the application of US on cells treated with folated eLipoDox.
Our current hypothesis as to why insonation produces no significant difference in KB cell viability when treated with folated eLipoDox is that the emulsion droplet vaporizes once endocytosed by the cell, even without US. This hypothesis is difficult to prove, but is supported by subsequent experiments showing the presence of bubbles in vials containing folated eLipoDox loaded using an ammonium sulfate buffer, but not with a potassium glutamate buffer. The Dox apparently forms a heterogeneous phase of fibers inside of the liposomes only when loaded using a multianionic buffer. It is our hypothesis that the Dox fiber provides a nucleation site for the PFC5 to form a gas phase that ruptures the liposome and causes the release of Dox independent of the application of US.
While US did not make a substantial difference in killing cells treated with folated eLipoDox, a higher percentage of KB cells were killed when treated with folated eLipoDox compared to an equivalent concentration of free Dox. Cell viability assays also show that the most cytotoxicity is produced by a combination of folate attached to the liposome, an emulsion droplet inside of the liposome, and Dox loaded in the liposome. Cytosolic delivery of Dox via folated eLipoDox did enhance the killing of MDR cells, reducing the viability to 60%, compared to 97% for MDR cells treated with free Dox at 7 µM.
None.
None.

©2017 Williams, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.