Journal of
eISSN: 2377-4282


Mini Review Volume 5 Issue 3
1Department of Chemistry, Sharif University of Technology, Iran
2Department of Chemistry, Iran University of Science and Technology, Iran
Correspondence: Behzad Pourbadiei, Department of Chemistry, Sharif University of Technology, Tehran, Iran, Tel 989374387564
Received: January 28, 2017 | Published: March 20, 2017
Citation: Pourbadiei B, Pyadar R, Mansouri F (2017) pH-Sensitive Nanoscale Polymers: Highly Efficient Systems for DOX Delivery in Cancer Treatment. J Nanomed Res 5(3): 00114. DOI: 10.15406/jnmr.2017.05.00114
Recently, many stimuli-responsive drug delivery systems based on biodegradable and biocompatible polymers have been introduced for controlled release of anticancer drug Doxorubicin. The main role of polymers in these systems is to provide high loading of Doxorubicin and then control the release of the drug in low pH-media. Polymeric micelles and nanoparticles such as magnetic particles, silica and graphene oxide covered with smart polymers include two main categories of Dox-controlled release systems. Herein, we review aforementioned systems which have been used for Doxorubicin release through different response of the system in terms of pH including: swelling or expansion of the polymeric chains, pH-sensitive bond cleavage and loss of electrostatic interactions between the drug and system. The mechanism of drug loading, drug release, advantages and disadvantages of each system are fully discussed.
In recent years, nanoscaled polymeric systems have shown amazing efficacy in drug delivery for cancer treatment due to their improved pharmacokinetics and biodistribution profiles via the enhanced permeability and retention (EPR) effect .1 Although, the EPR impact can only improve the accumulation of nanoparticles (NPs) in tumor tissues, the poor cellular internalization as well as insufficient intracellular drug release always has limited the dosages of anticancer drugs to the level below the therapeutic window, which hinders the efficiency of cancer chemotherapy .2To address this problem, environment-responsive delivery systems have demonstrated a fabulous pathway to improve the drug bioavailability.3 Accordingly, pH-responsiveness is a candidate to remove the problems in the cancer chemotherapy. pH values in different tissues and cellular compartments vary tremendously and that’s why pH-sensitivity is the most important factor in the improvement of the efficiency of cancer chemotherapy. For instance, the tumor extracellular environment has pH 6.5 which is more acidic than blood and normal tissues (pH 7.4), and endosome and lysosome are even more acidic (pH 5.0 - 5.5).4 To date diverse pH-responsive delivery systems have been designed and developed for pH-triggered drug delivery .5
Gong et al. 3 developed a pH-responsive polymer vesicle nanocarrier system for combined tumor-targeted delivery of an anticancer drug and superparamagnetic iron oxide (SPIO) nanoparticles (NPs). They designed these multifunctional polymer vesicles by heterofunctional amphiphilic triblock copolymers, that was, R(folate (FA) or methoxy)-poly(ethylene glycol)(Mw:5000)-poly(glutamate hydrozone doxorubicin)-poly(ethylene glycol) (Mw: 2000)-acrylate (i.e., R (FA or methoxy)-PEG114-P(Glu-Hyd-DOX)-PEG46-acrylate). The amphiphilic triblock copolymers were capable of self-assembly into stable vesicles in aqueous solution. They figured out that the long PEG segments were mostly segregated into the outer hydrophilic PEG layers of the vesicles, thereby providing active tumor targeting via FA. They figured out also that the short PEG segments were mostly segregated into the inner hydrophilic PEG layer of the vesicles, thereby making it possible to crosslink the inner PEG layer via the acrylate groups for enhanced in-vivo stability. They conjugated therapeutic drug, DOX, onto the polyglutamate segment, which formed the hydrophobic membrane of the vesicles using a pH-sensitive hydrazone bond to achieve pH-responsive drug release, while the hydrophilic SPIO NPs were encapsulated into the aqueous core of the stable vesicles, allowing for ultrasensitive magnetic resonance imaging (MRI) detection. For the SPIO/ DOX-loaded vesicles they reported a much higher r2 relaxivity value than Feridex, a commercially available SPIO-based T2 contrast agent, which was attributed to the high SPIO NPs loading level and the SPIO clustering effect in the aqueous core of the vesicles .2 (Figure 1).

Figure 1 Synthetic Figure of the amphiphilic triblock copolymers and the preparation process of the SPIO/DOX-loaded vesicles with cross-linked inner hydrophilic PEG layers.
Chen et al. 4 reported a multifunctional magnetic nanocarrier fabricated for synchronous cancer therapy and sensing. They programed this nanocarrier to display a response to environmental stimuli (pH value). They designed this system by coupling doxorubicin (DOX) to adipic dihydrazide-grafted gum arabic modified magnetic nanoparticles (ADH-GAMNP) via the hydrolytically degradable pH-sensitive hydrazone bond. They reported a mean diameter of 13.8 nm and the amount of DOX coupled was about 6.52 mg/g for the resultant nanocarrier, DOX-ADH-GAMNP. They claimed that their system exhibits pH triggered release of DOX in an acidic environment (pH 5.0) but is relatively stable at physiological pH (pH 7.4). In addition, they found out that both GAMNP and DOX possessed fluorescence properties when excited in the near-infrared region due to the two-photon absorption mechanism. Their experiments revealed that the coupling of DOX to GAMNP resulted in a reversible self-quenching of fluorescence through the fluorescence resonant energy transfer (FRET) between the donor GAMNP and acceptor DOX. They also realized that the release of DOX from DOX-ADH-GAMNP when exposed to acidic media indicated the recovery of fluorescence from both GAMNP and DOX. According to their results the change in the fluorescence intensity of DOX-ADH-GAMNP on the release of DOX could act as a potential sensor to sense the delivery of the drug. They also obtained the analysis of zeta potential and plasmon absorbance in different pH conditions and the results confirmed the pH sensitivity of the product. Their designed nanomedical system, which combined drug targeting as well as imaging, sensing and therapy at the same time, had the capability of simultaneous diagnosis and treatment of cancer .3 (Figure 2).
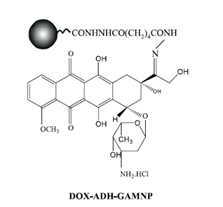
Figure 2 Fabrication of DOX-coupled GAMNP. The hydrazone bond formed between the COCH3 group of DOX and the hydrazide group of ADH-GAMNP could be effectively cleaved under acidic conditions around pH 5.0, which corresponds to that of endosomes/lysosomes in the cells.
Wang et al. 5 designed a tailor-made dual pH-sensitive polymer drug conjugate nanoparticulate system to overcome the challenges existed in cancer therapy due to drug resistance and inefficient cellular uptake. They reported the capability of their fabricated nanoparticle to reverse surface charge from negative to positive at tumor extracellular pH (∼6.8) to facilitate cell internalization. Furthermore, they observed that the significantly increased acidity in subcellular compartments such as the endosome (∼5.0) further promotes doxorubicin release from the endocytosed drug carriers. This dual pH-sensitive nanoparticle has showed enhanced cytotoxicity in drug resistant cancer stem cells, indicating its great potential for cancer therapy.4 (Figure 3).

Figure 3 Chemical Structure of the Dual pH-Responsive Polymer_Doxorubicin (DOX) Conjugate (PPC-Hyd-DOX-DA) and Schematic Illustration of Its pH Triggered Cellular Internalization and Intracellular Drug Release
Dhara et al.6 fabricated a temperature and pH dual responsive core–shell nanoparticles containing smart polymer shell coated on magnetic nanoparticles to be applied in cancer treatment as an anti-cancer drug carrier and cancer cell-specific targeting agent. They synthesized Dual-responsive poly(Nisopropylacrylamide)-block-poly(acrylic acid) copolymer via RAFT polymerization and then attached it to the amine-functionalized MNPs by EDC/NHS method. Moreover, they tethered folic acid to the surface of the nanoparticles to exhibit cancer-specific targeting properties and then they conjugated rhodamine B isothiocyanate to endow fluorescent property to the MNPs required for cellular imaging applications. According to their results, these nanoparticles were taken up specifically by HeLa cancer cells in comparison to normal cells. Their studies revealed that the outer polymer shell encapsulates large amount of anticancer drug Doxorubicin, and releases the anticancer drug preferably at pH 5.0 and temperature 37°C and also they reported the loading amount and entrapment efficiency as 23.0% and 74.4% respectively.5 (Figure 4).
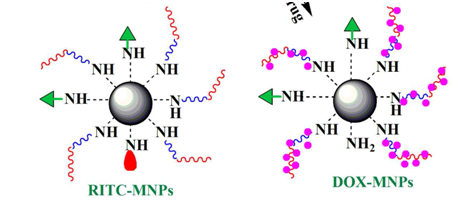
Figure 4 Proposed schematic presentation for the designing of polymer modified multifunctional magnetic nanoparticles.
Jiang et al.7 reported the designing and preparation of the pH-sensitive magnetic nanoparticles (MNPs) as targeted anti-cancer drug carriers. They coated MNPs by poly (acrylic acid) (PAA) to synthesize PAA@MNPs. They reported a size of 100 nm, good stability and superparamagnetic responsibility for PAA@MNPs (Ms 45.97 emu/g). They successfully loaded Doxorubicin (DOX) onto MNPs by electrostatic interaction. They reported that the drug loading content and loading efficiency were 26.4% and 88.1% respectively. In addition, their studies about release revealed that the drug-loaded carriers (MNPs-DOX) had significant pH-sensitivity. Also, studies showed that 75.6% of the loaded DOX was released at pH 4.0 within 48 h .6 (Figure 5).
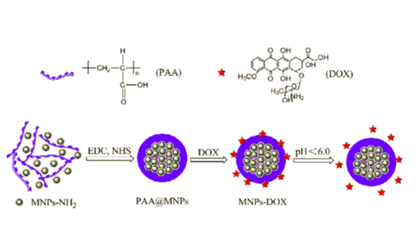
Figure 5 The preparation of DOX-loaded pH-sensitive, DOX was loaded onto PAA@MNPs via electrostatic interaction.
Yang et al.8 reported a dual-targeting drug delivery system based on multifunctionalized graphene oxide (GO) which was a pH-sensitive and controlled release system. Their target of this work was to improve the impact of targeted drug delivery and realize intelligently controlled release. They loaded Doxorubicin hydrochloride (Dox) onto the surface of this multi-functionalized GO via π–π stacking. They reported a saturation magnetization of 8.57 emu/g, The Dox loading capacity as high as 0.387 mg/mg in the case of the initial concentration of Dox at 0.238 mg/mL. Moreover, they claimed that their synthesized system’s release strongly depends on the pH value of environment.7 (Figures 6 & 7).
Wu et al. 7 designed a dually responsive nanocarrier for antitumor drug delivery. Their system was multilayer core-shell architecture. They prepared this nancarrier based on Fe3O4@SiO2 nanoparticles coated with mPEG-poly(l-Asparagine) (Figure 8).
In this work, they tethered imidazole groups (pKa∼6.0) to the side chains of poly(l-Asparagine) segments. They employed Fe3O4@SiO2 as a super-paramagnetic core, poly(l-Asparagine) as a pH-sensitive shell and mPEG as a hydrophilic corona. They successfully loaded doxorubicin (DOX) into the nanocarrier by combined actions of hydrophobic interaction and hydrogen bonding. According to the drug release studies they figured out that DOX release rate increased significantly as the ambient pH dropped from the physiological pH (7.4) to acidic (5.5). They attributed this to protonation and a change in hydrophilicity of the imidazole groups in the poly(l-Asparagine) segments .8
Drug delivery systems, sensitive to pH, have been loaded on diverse composite matrixes to deliver DOX drug (anti-tumor). DOX, due to possessing electronegative and protic groups, has the capability of making electrostatic and strong hydrogen bonds with polymer matrix. These attraction forces in lower values of pH are weakened and are removed and this gives rise to the drug delivery in the cancer cells.
These drugs have the benefit of the property in which cancer cells and tissues have lower values of pH. Moreover, the network structure of these polymeric matrixes causes to release and deliver drug with low speed and this is an advantage that gives rise to the controlled delivery of drug in lower values of pH. After complete delivery, due to making use of biodegradable polymer matrixes, these polymer matrixes are degraded and removed.
In recent years, because of breath taken increase in cancer all around the world, it is necessary the researches and studies on cancer drug delivery must be increased significantly to reach the point that these drugs be delivered in smart only on cancer tissues and cells.
None.
None.

©2017 Pourbadiei, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.