Journal of
eISSN: 2377-4282


Mini Review Volume 4 Issue 4
Derby University, UK
Correspondence: Matthew F Higgins, Derby University, Sport, Outdoor and Exercise Science, Kedleston Road, Derby, UK, Tel 44 1332 591743
Received: November 23, 2016 | Published: December 20, 2016
Citation: Higgins MF, Da Boit M (2016) Liposomal Nanotechnology - A New Frontier for Sport and Exercise Nutrition? J Nanomed Res 4(4): 00098. DOI: 10.15406/jnmr.2016.04.00098
There are many orally ingested nutrients which cannot be fully absorbed by the human body. For this reason scientists have been experimenting with different techniques to improve nutrient bioavailability. Among these techniques microencapsulation has been extensively used in industry in recent years, especially liposomal technology. Briefly, polar lipids are used to create spherical capsules, called liposomes, where solids, liquids or gaseous materials compounds can be entrapped. This technique is used to stabilize certain compounds in nutritional supplements and fortified foods, which would otherwise slowly degrade and lose their nutritional value, as well as improve their bioavailability. Although there has been limited research investigating nutrients that potentially might impact exercise performance (e.g. liposomal vitamin C and liposomal iron), there is currently no published evidence for the use of liposomal supplementation in this context. With the potential to augment nutrient bioavailability, further research should consider the application of liposomal formulations as a strategy to improve exercise performance.
Keywords: liposomes, nanotechnology, bioavailability, nutrition, sport and exercise, human performance
GI: Gastro–Intestinal; ND–CKD: Non–Dialysis Chronic Kidney Disease; URTI: Upper Respiratory Tract Infection; Hb: Haemoglobin; IV: Intravenous
The gastro–intestinal (GI) tract facilitates the digestion and adsorption of food, fluid and other biologically active compounds. Although human evolution evidently demonstrates the efficacy of this process, many molecules are (very) poorly adsorbed. Indeed, many vitamins and herbs have adsorption levels of ≤10%. As the active effect(s) of a nutrient is directly related to the quantity and rate in which the unchanged nutrient reaches the blood stream, the administrative route (i.e. oral, enteral or parenteral) and formulation (e.g. food, capsule, tablet, solution etc.) play significant roles in both of these outcomes for numerous nutritional supplements.1 This presents the obvious issue that nutrients or compounds which could have a variety of positive effects on the human body, particularly in specific therapeutic situations, are being prevented from doing so. For example, despite the reported multiple medicinal benefits of curcumin, its bioavailability is affected by poor absorption, extensive intestinal and hepatic metabolism and rapid elimination/clearance from the body.2 Moreover, the poor bioavailability of curcumin has been acknowledged as the likely major obstacle for greater use in humans.3 In an attempt to circumvent the reduced bioavailability of some nutrients, individuals might ingest larger than recommended doses. Such practice is relatively commonplace with consumption of ascorbic acid (vitamin C), although, due to poor absorption and subsequent osmotic effects, this can result in skin rashes or GI issues such as diarrhoea with intakes > 2000 mg/d.4,5 However, some evidence indicates intakes > 4000 mg/d are well tolerated in the general population.4 suggesting that, in those people suffering from the aforementioned side effects, bypassing any negative side effects of oral ingestion of vitamin C could provide significant therapeutic benefit.
Liposomal technology
Unsurprisingly, scientists have been exploring technological advancements with the aim of increasing nutrient bioavailability. Although a variety of encapsulation methods have been developed on both micro and nano–scales, liposomal technology (also called nanotechnology when liposomes are in the nanoscale range) has received significant interest from industry in recent years. With these technology solids, liquids or gaseous materials are packed into miniature capsules (membranes) that can subsequently release their contents at controlled rates under particular circumstances. Indeed, based on their ability to act as micro– or nanocarrier systems for the secure delivery of bioactive agents, liposomes are undergoing extensive research and development in, amongst others, the pharmaceutical, cosmetic and food industries.6,7
Liposomes, reportedly first described in the mid–1960s.8 are spherical (although shape is influenced by the structure of the different components) particles consisting of a membranous system formed by single (unilamellar) or multiple (multilamellar) lipid bilayers, resembling the lipid membrane of cells.9 which can be either small or large (Figure 1). These lipid bilayers are made of polar lipids which have a lipophilic and hydrophilic group. When hydrated in aqueous solution, polar lipids self–assemble, form bilayers and self–close into liposomes. Consequently, water soluble compounds are captured in the aqueous section, whilst lipid soluble materials collect in the lipid compartment.10 Due to having both lipophilic and hydrophilic groups in their structure, liposomes are unique in that they can be employed to encapture, transport and release water soluble, lipid soluble and amphiphilic materials, an advantage that not all encapsulation technologies possess.11 Liposomes are typically composed of lecithin (phosphatidylcholines) and kepalins (phosphatidylethanolamines), frequently containing negatively charged lipids such as phosphatidyl serine and phosphatidyl inositol. Additionally, sterols, such as cholesterol, and ceramides, such as sphingomyelin, are included.1 Furthermore, liposomes can be prepared using natural ingredients or molecules innate to the human body meaning they are highly biologically compatible and suitable for human consumption.11
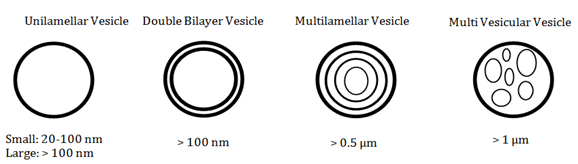
Figure 1 Types and typical sizes of different liposomes (*nm = nanometres, µm = micrometers; information based on 11.
Liposomal technology and nutrition
A key challenge in producing (fortified) foods and nutritional supplements is that certain compounds slowly degrade and lose their nutritional value. In order to improve their stability, different microencapsulation techniques can be used. Whilst liposomal technology was originally created for pharmaceutical purposes, in recent years its advantageous characteristics have been adopted by the food industry. In fact, a wide range of nutritionally relevant constituents such as essential oils, amino acids, antioxidants, enzymes, vitamins and minerals can be encapsulated in liposomes and nano–liposomes.11 For example, as explained by Schrooyen et al.12 vitamin C is added to a variety of food products to improve shelf life. For solid foods (e.g. biscuits, bread etc.) vitamin C is added by spray–cooling or spray–chilling and fluidized–bed coating, while for liquid products by liposomal encapsulation. Whilst vitamin C is added to some food products to enhance shelf life, it is also added for its functional and /or potential therapeutic benefits. Vitamin C is considered an anti–oxidant nutrient and essential cofactor for a variety of enzymes involved in physiological processes. Despite being essential for many functions, vitamin C has low bioavailability and cannot be synthesized de novo by the body.13 thus it has to be introduced through the diet.14
Although nowadays most western civilizations have easy access to sources of vitamin C such as fruit and vegetables, the tendency to use microwaved food has contributed to an increased incidence of scurvy (severe deficiency of vitamin C).15 A lack of vitamin C intake has also been associated with the incidence of chronic diseases such as obesity.16,17 whilst vitamin C supplementation has been shown to improve triglyceride accumulation.18 and endothelial function.19 In order to improve vitamin C bioavailability and thus boost its beneficial effects, researchers have started to consider liposomal formulations as a good alternative to standard oral vitamin C supplementation. The first study to describe the pharmacokinetics of different single doses (5, 20, and 36 g) of liposomal vitamin C, versus ‘standard’ oral vitamin C (5 g), was conducted by Hickey and colleagues.20 The authors reported that the highest peak plasma level (~ 400 uM/L) was achieved with 36 g, peaking after ~5–6 hours post–ingestion. A more recent study investigated the efficacy of liposomal vitamin C and its effects on ischemia–reperfusion injury.21 Oral placebo or 4 g of vitamin C via oral unencapsulated, oral liposomal, or intravenous delivery were administered. Liposomal vitamin C produced higher blood levels of vitamin C than those elicited by unencapsulated vitamin C, but less than that following intravenous vitamin C administration (Figure 2). Moreover all treatments, except placebo, provided equal protection from ischemia–reperfusion injury.21

Figure 2 Plasma concentrations of vitamin C (ascorbic acid) before (time = 0 minutes) and after supplementation. (A) All treatments. (B) All treatments excluding intravenous administration. *P < 0.001 vs. all other treatments; # P < 0.001 vs. unencapsulated oral and placebo; and ^P < 0.001 vs. placebo. (Reproduced in full with permission from 21).
As iron deficiency is a common cause of anaemia in non–dialysis chronic kidney disease (ND–CKD) and controversy still exists about the optimal mode of iron therapy, Pisani et al.22 recently evaluated whether liposomal iron improved anaemia in ND–CKD patients. Using a randomized experimental approach, 99 patients with CKD and iron deficiency anaemia were assigned to receive 30 mg/day oral liposomal iron (LI; n=66) or intravenous iron gluconate (IV: total dosage = 1000 mg; n=33) for 3 months. After three months both LI (5.6%) and IV groups (9.3%) had significantly increased Hb levels compared with baseline with no difference between treatments. However, the trajectory of increase was more pronounced for the IV group which had significantly higher Hb at the end of months 1 and 2 compared to LI. In contrast, although no ‘serious’ adverse effects were reported, the proportion of participants who experienced at least one treatment related adverse event was substantially lower for LI (~3.1%) compared to IV (~35%). As iron deficiency and iron deficiency anemia are major concerns for the health and performance of male and female elite athletes.23 and the fact that liposomal iron has shown improvements of exercise associated anemia in a vitro model.24 further research examining liposomal iron in athletic individuals is warranted.
Liposomal technology and sport and exercise nutrition
The antioxidant potential of vitamin C to improve sport and exercise performance, either directly or indirectly, has been extensively researched. Due to the volume of training/physiological stress encountered elite athletes tend to suffer more from upper respiratory tract infections (URTIs) than the general population.25 In populations undergoing severe physical stress, including athletes, vitamin C has been shown to be effective in reducing not only symptoms and duration, but also incidence of colds.26 making vitamin C a useful strategy in preventing training interruptions. Moreover, vitamin C has been reported to ameliorate exercise–induced bronchoconstriction.27 enhance carnitine synthesis.28 and to regulate fatty acid utilisation during exercise.29
Although current approaches to oral vitamin C supplementation (e.g. 500 mg oral capsules) might provide some benefit during exercise.29 the potential effects of liposomal vitamin C on exercise performance is yet to be explored. Moreover, there appears to be no research with respect of liposomal technology applied to sport nutrition generally. This is somewhat surprising as this technique could be used for oral administration of other nutrients valuable to sport and exercise performance, which could be more extensively studied upon improvement in their bioavailability. For instance, phenols, known for their very low bioavailability but high anti–oxidant and anti–inflammatory characteristics, have also been demonstrated to be useful in improving exercise performance. In fact, there is evidence that resveratrol, quercetin and catechins have positive effects on aerobic capacity.30–32 and curcumin on preventing muscle damage.33 Moreover, liposomal phenols are already extensively utilised in cancer treatment: resveratrol and catechins liposomes to target cancer cells.34,35 liposomal resveratrol combined with quercetin to improve pre–cancerous/cancerous skin lesions.36 and liposomal curcumin and resveratrol to prevent cancer.37 Therefore, the already existing beneficial effects of these phenols might be augmented by the use of liposomal formulations within a sport and exercise context.
Liposomal technology is a promising adjunct that could address limitations regarding the bioavailability and absorption of a plethora of nutrients/compounds. As such, the use of liposomal technology has numerous important applications in both sport and exercise and health settings. However, despite its potential, research on oral liposomal formulations in these contexts is scarce. To the best of our knowledge only a few studies have explored the absorption and effects of oral nutritional liposomal formulations on human health and no research has been conducted on exercise performance in humans. Further research looking at liposomal nutrients for sport nutrition is therefore warranted.
Dr. Matthew F. Higgins and Dr. Mariasole Da Boit are currently undertaking research which aims to examine the effects of liposomal vitamin C on sport and exercise performance. This project is co–funded by a grant from Innovate UK (https://www.gov.uk/government/organisations/innovate–uk) and Sure Screen Diagnostics Limited (https://www.surescreen.com/).
None.

©2016 Higgins, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.