Journal of
eISSN: 2377-4282


Research Article Volume 4 Issue 4
International Institute of Medicine and Science, USA
Correspondence: Alain L Fymat, International Institute of Medicine and Science, California, USA, Tel (760) 485-9149
Received: November 14, 2016 | Published: December 12, 2016
Citation: Fymat AL (2016) Recent Developments in Nanomedicine Research. J Nanomed Res 4(4): 00096. DOI: 10.15406/jnmr.2016.04.00096
Nanomedicine Drugs Cited
In 1995, approval of the very first nanotherapeutics product (Doxil) is generally viewed as the dawn of nanomedicine for human use. A generally accepted definition of nanomedicinerefers to highly specific medical interventions at the molecular scale using nanotechnology methods, products and devices for curing disease or repairing damaged tissues (such as bone, muscle, or nerve). In early 2003, the European Science Foundation (ESF) launched its foresight study titled “Scientific Forward Look on Nanomedicine”1 In 2004, the U.S. National Institute of Health (NIH) published its “Roadmap (now Common Fund) of the Nanomedicine Initiative” .2 This latter program began in 2005 with a national network of eight Nanomedicine Development Centers. Now, ten years into this program, four of these initial centers were judged to be best positioned to effectively apply their findings to translational studies. Also beginning in 2004, the U.S. National Cancer Institute (NCI} initiated the “Alliance for Nanotechnology in Cancer“ 3 to spearhead the integration of nanotechnology into biomedical research through the coordinated effort of a network of investigators from diverse institutions and organizations. It includes:
The Centers of Cancer Nanotechnology Excellence (CCNE)
To integrate discovery and tool development for nanotechnology applications into clinical oncology. CCNEs link physical scientists, engineers, and technologists working at the nanoscale with cancer biologists and oncologists.
The Cancer Nanotechnology Platform Partnerships (CNPP)
To engage in directed product-focused research that aims to translate cutting-edge science and technology into the next generation of diagnostic and therapeutic tools. These platforms serve as core technologies for a wide array of applications.
The Cancer Nanotechnology Training Centers (CNTC)
To educate and train researchers from diverse fields in the use of nanotechnology-based approaches to advance understanding of cancer biology and create new methods and tools for the prevention, diagnosis and treatment of cancer.
The Pathways to Independence Awards in Cancer Nanotechnology Research (PIA-CNR)
To grant awards to post-doctoral scientists working on cancer nanotechnology to facilitate a timely transition from a mentored postdoctoral research position to a stable independent research position with the overall goal of maintaining a strong pool of new talented investigators focused on research in cancer nanotechnology.
The Nanotechnology Characterization Laboratory (NCL)
To perform preclinical efficacy and toxicity testing of nanoparticles in a joint effort of NCI, NIST (the U.S. National Institute of Standards and Technology) and the FDA (the U.S. Food and Drug Administration). By providing critical infrastructure and characterization services to managerial providers, the NCL accelerates the transition of basic nanoscale particles and devices into clinical applications.
The Physical Sciences-Oncology Centers Program (PS-OCP)
A collaborative network of 12 institutions bringing together cancer biologists, oncologists, and researchers from disciplines in the physical sciences to address major questions and barriers in cancer research. Several centers are using nanotechnology-based approaches, including one center that is using fluidity and microfloppy devices to model the tumor environment in three-dimensions.
By 2013, the number of approved products had reached 54 in all, with another 150 in various stages of clinical trials. A PubMed search on articles relating to nanomedicine shows 7,400 hits over 10 years, of which 1,874 were published in 2013 alone. Similarly, the US patent office database shows 409 patents (since 1976) that were granted in nanomedicine, with another 679 applications awaiting approval. However, despite substantial research activity and funding, and notwithstanding the wide use of nanotechnology in medicine (in vitro diagnostics,in vivoimaging, injectable therapeutics), nanomedicine has not yet translated to clinical success nor revolutionized treatment paradigms as may have been envisaged earlier. In particular, no molecular machine or nanorobot has yet entered clinical trials, although research in these areas is picking up pace. I will describe below the most noteworthy recent research developments in nanomedicine after first analyzing some of the applications of nanomedicine and considering the major obstacles that hinder the up scaling of nanomedicine products.
Nanomedicine is the medical application of nanotechnology ranging from the medical applications of nanomaterials, to nanoelectronic biosensors, and even possible future applications of molecular nanotechnology. Because of their small size, nanoscale materials and devices can interact readily with biomolecules both inside and on the surface of cells with the potential to detect disease and deliver treatment in ways previously unimagined. Nanoparticles are promising tools in that they and provide can facilitate research, improve molecular imaging, early detection and provide diagnosis, prevention, control, and even treatment of cancer
Cellular and molecular dynamics: Nanotechnology offers tools to monitor individual cells and molecules and track their movements in their environment. It enables researchers to study, monitor, and manipulate the multiple systems that go awry (e.g., in the cancer process). Nanoparticles can also be used for intracellular targeting, intracellular drugs, mRNA applications, and gene silencing applications.
Molecular imaging improvement and early pathology detection: Nanotechnology can help spot cancer in its earliest stages. Detection of biomarkers using nanotechnology evidences features that are undetectable through conventional imaging. In addition, photo luminescent nanoparticles may allow oncologists to visually discriminate between cancerous and healthy cells.
Disease prevention and control: Advances driven by the several national and international cancer initiatives, particularly in proteomics and bioinformatics will enable researchers to identify markers of cancer susceptibility and precancerous lesions. Nanotechnology can then be used to develop devices that indicate when those markers appear in the body and deliver agents to reverse premalignant changes or even kill those cells that have the potential to become malignant.
Drug delivery and therapeutics: Because of their diverse capabilities, nanoscale devices can contain both targeting and therapeutic agents (in both single and multi-drug approaches). They can deliver high drug levels in several situations, including: anticancer drugs at the tumor site that can also increase chemotherapeutic efficacy; antihypertensive drugs; cerebrovascular drugs that can penetrate the blood brain barrier (BBB). Nanoscale devices also offer the opportunity to develop new approaches to therapy, combine a diagnostic or imaging agent with a drug, and determine whether the drug acts on its intended target. "Smart" nanotherapeutics may provide the ability to "time" the release of any given drug or to deliver multiple drugs sequentially in a timed manner or at several locations in the body.
Commercial applications
These encompass a wide array of fields including the following:
Pharmaceutical Industry: Formulations of anti-cancer, anti-hypertensive and anti-cerebral drugs; drug delivery systems; in vivo imaging; new therapies; and physiochemical, pharmacological, and immunological characterization of nanoparticles. Also included are: Pharmacokinetics and pharmacodynamics of drugs associated with nanoparticles; theranostic nanoparticles and their applications; and biodegradable nanoparticle carriers.
Neuro-electronic interfaces.
Nano electronic-based sensors.
Molecular nanotechnology: in which it is speculated that cell repair machines could revolutionize medicine and the medical field
Nanooncology: Unique characteristics of nanoparticles make them highly attractive for various applications in oncology. They are able to function as (a) carriers for chemotherapeutic drugs to increase their therapeutic index and lower their toxicity; (b) therapeutic agents in photodynamic, gene, and thermal therapy, as well as (c) molecular imaging agents to detect and monitor cancer progression. Several nanoparticle-based agents for cancer therapy and diagnostics have been approved by the U.S. FDA, more are in clinical trials, and even more are in the discovery and early development stages in academic and industry laboratories. Topics of study in Nanooncology include but are not limited to: photodynamic therapy (PDT); targeted photo thermal therapy (TPT); thermal ablation of cancer; carriers (liposomal, solid lipid, polymeric, mesoporous silica, and inorganic nanoparticles). For a more detailed discussion, refer to.4,5
Major Obstacles to Scaling up Nanomedicine Products
Nanomedicine is taking long to bear fruits for several reasons, including:
Uncertainty over the environmental consequences of the use of nanotechnology
This uncertainty arises, for example, in handling during manufacture. It has necessitated more elaborate safety testing that consequently led to a longer pre-clinical phase of development and, thus, to higher costs of development.
Required stringent safeguards for handling nanoparticles and their perceived toxic effects
Nanotoxicology must take a more central role in product development. Current problems for nanomedicine involve understanding the issues related to toxicity and environmental impact of nanoscale materials.
Insufficient control over drug efflux from nanocarriers
This is not a trivial task. Other routes of administration other than intra-venous injections must also be explored.
Tortuous scaling up of nanomedicine products
This has cost time and money. The return on financing of such projects (with long commercialization times) may be marginal for many current nanomedicine concepts.
Needed truly multidisciplinary research
Such multidisciplinary research is needed to drive the solutions to current medical needs. It requires the active participation from clinician scientists at every stage of the process. Of note, several multidisciplinary nanomedicine centers have been set up worldwide (for example at Northwestern University, University of California at San Diego, and Nanyang Technological University, to name a few).
Nanomedicine is a large industry, with nanomedicine sales reaching $ 6.8 B in 2004, and with over 200 companies and 38 products worldwide. A minimum of $ 3.8 B is invested every year in nanotechnology. As the nanomedicine industry continues to grow, it is expected to have a significant impact on the economy. Nonetheless, in the final analysis, only with demonstrable and superior patient benefits, and overall cost reductions in healthcare management, can nanomedicine make bigger inroads.
Recent Developments in Nanomedicine Research
In vivo imaging
Nanoparticle contrast agents: Images such as ultrasound and magnetic resonance imaging (MRI) have a favorable distribution and improved contrast.6
Quantum dots: Quantum dots are nanoparticles with quantum confinement properties (such as size-tunable light emission), which, when used in conjunction with MRI, can produce exceptional images of tumor sites. These nanoparticles are much brighter than organic dyes and only need one light source for excitation. “Fluorescent quantum dots” could produce a higher contrast image and at a lower cost than today's organic dyes used as contrast media. The downside is that quantum dots are usually made of quite toxic elements. As an example, quantum dots of cadmium selenide glow when exposed to UV light. When injected, they seep into cancer tumors. The surgeon can see the glowing tumor, and use it as a guide for more accurate tumor removal.
Drug delivery
Engineered nanoscale devices: Nanoscale devices can be engineered to aid the delivery of life-saving drug treatments (cancer, neurological diseases, brain disorders, etc.) at the affected sites. Such minute devices have the potential to be engineered to efficiently and more safely deliver drug treatments directly to the location of diseased cells while helping avoid harm to healthy cells that fall victim to toxic drugs administered by conventional means. A research project led by Dr. Dayong Jin.7 at the University of Technology in Sidney with colleagues from Macquarie University, the University of Wollongong, and the National University of Singapore has produced a library of 800 different and uniquely shaped hybrid nanocrystals, formed from ordered atom clusters, that act as new tools or molecular tags enabling and aiding targeted drug delivery.7 These new nanocrystals are multifunctional and able to be multi-tasked to do different things simultaneously. For example, they can be designed to exhibit optical, magnetic and chemical responses allowing higher spatial resolution, multiple modalities imaging of a disease. Now, fabrication of nanoparticles can be precisely controlled to create different shapes and sizes, allowing the assessment of the drug impact along its propagation path within the body. It could also assist in the development of clearer diagnostic bio-imaging modalities such as MRI and CT scans.
Bioavailability-improved nanoscale particles and molecules: Nanoscale particles and molecules can also be developed to improve drug bioavailability, i.e., the presence of drug molecules where they are needed in the body and where they will do the most good. Drug delivery focuses on maximizing bioavailability both at specific places in the body and over a period of time. It can be achieved by molecular targeting by nano-engineered devices targeting the molecules and delivering drugs with cell precision. The basic process to use drug delivery involves at least three steps: (a) Encapsulation of the drugs; (b) Successful delivery of said drugs to the targeted region of the body; and (c) Successful release of that drug there.
Several nanoparticles are employed
Nutshells (120 nm in diameter coated with gold): Can deliver drugs to kill cancer tumors in mice. The nutshells can be targeted to bond to cancerous cells by conjugated antibodies or peptides to the anopheles' surface. By irradiating the tumor with an infra-red (IR) laser, which passes through flesh without heating it, the gold is heated sufficiently to cause death to the cancer cells (Jennifer West, Rice University).8
Platelet-coated nanoparticles (~ 100 nm in diameter): Can deliver drugs to targeted sites in the body, particularly injured blood vessels, as well as organs infected by harmful bacteria. By delivering the drugs to the sites where they are needed, these nanoparticles can greatly increase their therapeutic effects by directly depositing a much higher dose of medication specifically to diseased areas without saturating the entire body with drugs. This principle has broad implications for targeted therapy for other diseases such as cancer and neurological disorders.
There are several medical advantages to these nanoparticles
Gelatin nanoparticles for delivering multiple drugs to the brain: Gelatin is biocompatible, biodegradable, and classified as “generally recognized as safe” by the FDA. Once administered, the gelatin nanoparticles target damaged brain tissue thanks to an abundance of gelatin-munching enzymes produced in injured regions. They also have neuroprotective effects. To test them as a drug-delivery system, researchers at the Universities of Illinois and South Korea administered intranasally the gelatin nanoparticles laced with the drug osteopontin (OPN) in a rat ischemic stroke model.9 In rats, OPN can help reduce inflammation and prevent brain cell death if administered immediately after a stroke. By lacing gelatin nanoparticles with OPN, the treatment window can be extended to six hours or even more as they show the same efficacy rate as giving them OPN alone. After one hour-70 percent recovery of dead volume in the brain was observed. Overcoming the difficulty of delivering therapeutic agents to specific regions of the brain presents a major challenge to the treatment of most neurological disorders. However, if drug substances can be transferred along the olfactory nerve cells, they can bypass the BBB (a biological fence that prevents the vast majority of drugs from entering the brain through the bloodstream). In this regard, the tiny gelatin particles have a huge benefit in that they can be administered nasally - a noninvasive and direct route to the brain. This allows the drug to bypass the BBB. Administered in the same manner, gelatin nanoparticles can help deliver much therapeutics to the brain to more effectively treat a variety of brain injuries and neurological diseases in stroke and other victims. They will be most effective in delivering drugs that cannot otherwise cross the BBB. In addition, they can be used for drugs of high toxicity or a short half-life. In particular, stroke victims could have more time to seek treatment that could reduce harmful effects on the brain resulting from delayed treatment (say of several hours).
Shape-shifting engineered nanoparticles: Nanoparticles can also be engineered so as to respond to biological molecules by changing shape to gain access to diseased tissue.10 These shape-shifters are made of minuscule chunks of metal with strands of DNA attached to them. This targeted molecular delivery system uses modular nanoparticles whose shape, size and chemistry can be altered by the presence of specific DNA sequences. The nanoparticles float around harmlessly in the blood stream until a DNA strand binds to a sequence of DNA known to be a marker for cancer. When this happens, the particle changes shape then carries out its function: target the cancer cells, expose a drug molecule to the cancerous cell, and tag the cancerous cells with a signal molecule. This approach can theoretically be imbedded in personalized nano medical treatments, further tailoring the particles to deliver drugs to specified tumors and nowhere else.
Kinase inhibitors in nanoparticle formulation: Efforts to apply nanotechnology in cancer have focused almost exclusively on the delivery of cytotoxic drugs to improve therapeutic index. There has been little consideration of molecularly targeted agents, in particular kinase inhibitors, which can also present considerable therapeutic index limitations. Ashton and colleagues .11 have developed accurin polymeric nanoparticles that encapsulate the clinical candidate AZD2811, an Aurora B kinase inhibitor, using an ion-pairing approach.11 Accurins increase biodistribution to tumor sites and provide extended release of encapsulated drug payloads. AZD2811 nanoparticles containing pharmaceutically acceptable organic acids as ion-pairing agents displayed continuous drug release for more than 1 week in vitro and a corresponding extended pharmaco dynamic reduction of tumor phosphorylated histone H3 levels in vivo for up to 96 hours after a single administration. A specific AZD2811 nanoparticle formulation profile showed accumulation and retention in tumors with minimal impact on bone marrow pathology, and resulted in lower toxicity and increased efficacy in multiple tumor models at half the dose intensity of AZD1152, a water-soluble pro-drug of AZD2811. These studies demonstrated that AZD2811 can be formulated in nanoparticles using ion-pairing agents to give improved efficacy and tolerability in preclinical models with less frequent dosing. Accurins specifically, and nanotechnology in general, can increase the therapeutic index of molecularly targeted agents, including kinase inhibitors targeting cell cycle and oncogenic signal transduction pathways, which have to date proved toxic in humans. A phase 1 clinical trial is the next step for this nanomedicine approach, and additional preclinical studies will reveal whether such nanoformulations can improve the tolerability and efficacy of the broader class of molecularly targeted cancer therapeutics, including cell cycle inhibitors.
Nucleic acids as cancer biomarkers: Researchers at Wake Forest Baptist Medical Center.12 have developed a new technology to detect disease biomarkers in the form of nucleic acids, the building blocks of all living organisms (Figure 1). This is a potential first-line, non-invasive diagnostic to detect anything from cancer to the Ebola virus. The test could eventually be performed using a few drops of blood from a single finger prick. Nucleic acids consist of chains or sequences of bases stretching from just a few to millions of elements long. The exact order in which these bases are found, even over short distances, is strongly tied to their functions, and therefore can be used as direct indicators of what is going on inside cells and tissues. For example, one family of these nucleic acids known as micro RNAs (miRNAs) are only about 20 bases long, but can signal a wide range of diseases, including cancer. Although miRNA biomarkers have been studied for years, one problem has been their accurate detection because they are so short as many technologies had real difficulty identifying them. In this new technique, nanotechnology is used to determine whether a specific target nucleic acid sequence exists within a mixture and to quantify it through a simple electronic signature. If present, it forms a double helix with the probe just designed to provide a clear signal. By simply counting the number of signals, one can determine the extent of the surrounding target. The researchers first demonstrated that the technology could effectively identify a specific sequence among a background of competing nucleic acids, and then applied their technique to one particular miRNA (mi-R155) known to indicate lung cancer in humans. They showed that the approach could resolve the minute amount of miRNAs that can be found in patients. Next steps will involve expanding the technology to study clinical samples of blood, tissue or urine.

Figure 1 The Hybridization of a Target Nucleic Acid with a Synthetic Probe Molecule Enables Discrimination between Duplex and Single-Stranded Molecules with High Efficacy. [From: Wake Forest Baptist Medical Center.12].
Nanoparticles and Cancer Treatment
For a lengthier discussion and other examples of nanoparticles and cancer, the reader is referred to a companion article by this author.5
Tumor/cancer imaging and eradication
New nanoengineered materials being developed might be effective in treating illnesses and diseases such as cancer. Because nanoparticles have high surface area to volume ratio, many functional groups could be attached to a nanoparticle, which can then seek and bind to certain tumor cells. The small size of nanoparticles allows them to preferentially accumulate at tumor sites (because tumors lack an effective lymphatic drainage system). It is possible to manufacture multifunctional nanoparticles that would detect, image, and then proceed to treat a tumor.13
One example would be Kanzius RF energy to obliterate tumors. The technology attaches microscopic nanoparticles to cancer cells and then “cooks” tumors inside the body, with radio-waves that heat only the nanoparticles and the adjacent (cancerous) cells. Sensor test chips containing thousands of nano wires, able to detect proteins and other biomarkers left behind cancer cells, could enable the detection and diagnosis of cancer in the early stages from a few drops of a patient's blood .14
Virtual biopsy and treatment of brain metastatic tumors
Differential diagnosis of image enhancements in brain MRI remains a significant problem. This problem may be difficult to resolve without biopsy, which can be often dangerous or even impossible. Such MRI enhancement(s) can result from metastasis of primary tumors such as lung or breast, radiation necrosis, infections, or a new primary brain tumor (glioma, meningioma). Neurological symptoms are often the same on initial presentation. To develop a more precise noninvasive MRI diagnostic method, a new class of poly (β-l-malic acid) polymeric nanoimaging agents (NIAs) was developed. The NIAs-carrying attached MRI tracers are able to pass through the BBB and specifically target cancer cells for efficient imaging.
A qualitative/quantitative MRI virtual biopsy method is based on a nanoconjugate-carrying MRI contrast agent gadolinium-DOTA and antibodies recognizing tumor-specific markers and extravasating through the BBB. In newly developed double tumor xenogeneic mouse models of brain metastasis, this noninvasive method allowed differential diagnosis of HER2- and EGFR-expressing brain tumors. After MRI diagnosis, breast and lung cancer brain metastases were successfully treated with similar tumor-targeted nanoconjugates carrying molecular inhibitors of EGFR or HER2 instead of imaging contrast agent. The treatment resulted in a significant increase in animal survival and markedly reduced immunostaining for several cancer stem cell markers. Novel NIAs could be useful for brain diagnostic MRI in the clinic without currently performing brain biopsies. This technology shows promise for differential MRI diagnosis and treatment of brain metastases and other pathologies when biopsies are difficult to perform.
Lung cancer and chronic obstructive pulmonary disease
Nanoparticles can penetrate deep into lung compartments that are otherwise impossible to reach. This capability will enable the treatment of chronic obstructive pulmonary disease (COPD), which is the progressive, irreversible obstruction of lung airways causing almost 1 in 10 deaths today. Treatment of COPD and lung cancer commonly involves chemotherapeutics and corticosteroids misted into a fine spray and inhaled, enabling direct delivery to the lungs and quick medicinal effect. However, because the particles produced by today's inhalers are large, most of the medicine is deposited in the upper respiratory tract. Researchers at the Harvard University and the Malaysia Institute for Innovative Technology (MIIT) have jointly developed “smart” nanoparticles that deliver appropriate levels of diagnostic and therapeutic agents to the deepest, tiniest sacs of the lung, a process potentially assisted by the use of magnetic fields.15 The study builds on Harvard's extensive expertise in biokinetics (the determination of how to administer medicines to achieve the proper dosage to impact target cells and assess the extent to which drugs-loaded nanoparticles pass through biological barriers to different organs). The study also builds on decades of experience studying the biology of macrophages - large, specialized cells that recognize, engulf and destroy target cells as part of the human immune system. Manipulating immune cells represents an important strategy for treating lung diseases like COPD and lung cancer, as well as infectious diseases including tuberculosis and listeriosis.
Every day, humans breathe 20,000 liters of air loaded with bacteria and viruses. The world's deadliest epidemic - an outbreak of airborne influenza in the 1920s- killed tens of millions of people. Inhaled nanomedicine holds the promise of helping prevent and treat such problems in the future, reaching the target area more swiftly than if administered orally or even intravenously. Meanwhile, COPD affects over 235 million people worldwide and is on the rise, with 80% of cases caused by cigarette smoking. Exacerbated by poor air quality, COPD is expected to rise from 5th to 3rd place among humanity's most lethal health problems by 2030.
Pancreatic cancer
Nanoparticles combine photodynamic and molecular therapies against pancreatic cancer. A nanoparticle drug-delivery system that combines two complementary types of anticancer treatment could improve outcomes for patients with pancreatic cancer and other highly treatment-resistant tumors while decreasing toxicity. Researchers at the Wellman Center for Photo medicine at Massachusetts General Hospital (MGH) have demonstrated how such a nanomedicine approach that combines a drug and photodynamic therapy (PDT) reduced a thousand-fold the dosage of the molecular therapy drug required to suppress tumor progression and metastatic outgrowth in an animal model.16 (PDT is the use of light to trigger a chemical reaction with a molecular therapy drug targeted against common treatment resistance pathways.) This new optically active nanoparticle is able both to achieve tumor photo-damage and to suppress multiple escape pathways, opening new possibilities for synchronized multidrug combination therapies and tumor-focused drug release.
Ocular cancer
Researchers at the University of Michigan Kellogg Eye Center have developed a new nanoparticle that uses a tumor cell’s protective mechanism against itself, short-circuiting tumor cell metabolism and killing tumor cells.17 It uses a semiconducting nanoparticle with an attached platinum electrode to drive the synthesis of an anti-cancer compound when illuminated by light. The nanoparticle mimics the behavior of NADPH oxidase, an enzyme used by immune cells to kill tumor cells and infectious agents. Since tumor cells typically use NADPH to protect themselves from toxins, the more NADPH they synthesize for protection, the faster they die. This treatment offers many advantages: (a) The nanoparticle produces about 20 million toxins per hour in each cell; (b) It is activated by light so it can be turned on and off simply by exposing it to the correct color of visible light; and (c) It has the potential to be used for multiple applications in ophthalmology and other disciplines.
Chemotherapy
Tracking the path of chemotherapy drugs in real time and at the cellular level could revolutionize cancer care and help sort out why two patients might respond differently to the same treatment. Up until now, this was accomplished, admittedly in a limited way, by organic dyes (that faded quickly) and by toxic elements (particularly, metals). However, researchers at the Ohio State University.18 have devised an organic technique using nanotechnology to light up a common cancer drug doxoribucin (although any other cancer drug could be employed) so they can see where the chemotherapeutic drug goes and how long it takes to get there. They first created a luminescent molecule (a peptide made of two amino acids) and hitched that light to the cancer medication so that it revealed the drug pathway and arrival within the cells.9 Importantly, as it enters the cancerous site, that peptide easily coexists with human cells and leaves them harmless. Composed of natural amino acids, the nanoparticle is inherently biocompatible. This work was completed in the laboratory and is currently being tested on animals. This methodology could be helpful in the field of personalized medicine wherein the better understanding of the complex interplay of cells and drugs is critical to the development of treatments that are finely tuned for individual patients.
Nanoparticles and cardiovascular disease
Cardiovascular disease (arteriosclerosis) is often caused by endothelial cell (EC) dysfunction and atherosclerotic plaque formation at predilection sites. These pathological deposits form in the arteries leading to vascular stenosis. Strokes and heart attacks are a frequent outcome due to the resultant insufficient blood flow. In a normal situation, the ECs which line the blood vessels play an important role as they produce nitrous oxide and regulate the expansion of the vessels and the blood pressure. Arteriosclerosis is usually “corrected” by surgically removing the vascular deposits and inserting a vascular support (stent) to correct the bottleneck in the crucial blood supply. Unfortunately, surgical procedures of plaque removal cause irreversible damage to the EC layer, inducing impairment of vascular function and restenosis wherein these areas become blocked with deposits once again. In the usual case where a supporting vascular stent was placed, in case of restenosis, it becomes necessary to remove/replace the stent (assuming the patient did not pre-decease the operation).
Researchers from the Universities of Bonn and Munich have now developed a method that gets to the root of the problem (the restoration of the original condition of healthy ECs) by guiding replacement cells to diseased vascular segments using nanoparticles.19 In this potentially curative approach, radially symmetric re-endothelialization of vessels is performed after their mechanical denudation. For this purpose, a combination of nanotechnology with gene and cell therapy was applied to site-specifically re-endothelialize and restore vascular function. Together with the gene, tiny nanoparticles with an iron core are introduced. The iron changes the properties of the ECs rendering them magnetic. They are then guided by a specially-designed ring-shaped magnet to ensure that the replacement cells line the blood vessel evenly while exerting their curative effect. The researchers used complexes of lentiviral vectors and magnetic nanoparticles (MNPs) to over express in the ECs the vasoprotective gene endothelial nitric oxide synthase (eNOS, an enzyme that is an essential precondition for the full restoration of the original function of the ECs as it stimulates nitric oxide production in the endothelium like a turboloader). The MNP-loaded and eNOS-over expressing cells were magnetic, and by magnetic fields they could be positioned at the vascular wall in a radially symmetric fashion even under flow conditions. Thus, the combination of MNP-based gene and cell therapy with custom-made magnetic fields enables circumferential re-endothelialization of vessels and improvement of vascular function (Figure 2). Nonetheless, while the method has been successfully tested on mice whose carotid artery endothelial cells were injured, much research remains to be done prior to its use in humans.
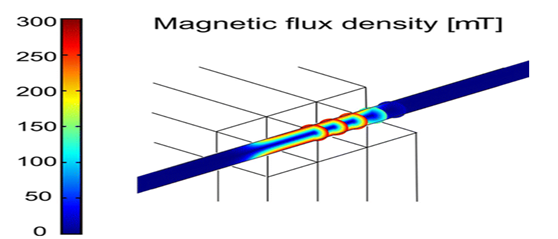
Figure 2 Vascular Repair by Circumferential Cell Therapy using Magnetic Nanoparticles and Tailored Magnets.19
Nanoparticles in surgery
At Rice University, a “flesh welder” is used to fuse two pieces of chicken into a single piece. The two pieces of chicken are placed together touching. A greenish liquid containing gold-coated nanoshells is dribbled along the seam. An IR laser is traced along the seam, causing the two sides to weld together. This could solve the difficulties and blood leaks caused when the surgeon tries to restitch the arteries that have been cut during a kidney or heart transplant. The flesh welder could weld the artery perfectly.20
Nanoparticles in the treatment against antibiotic-resistant superbug
Antibiotic-resistant bacteria such as Salmonella, E. Coli and Staphylococcusinfect some two million people and kill at least 23,000 people in the United States each year. Efforts to thwart these so-called "superbugs" have consistently fallen short due to the bacteria's ability to rapidly adapt and develop immunity to common antibiotics such as penicillin. Previous research has shown that metal nanoparticles created from gold and silver among other metals can be effective at combating antibiotic resistant infections, but can indiscriminately damage surrounding cells as well. Researchers at the University of Colorado in Boulder and the BioFrontiers Institute.21 have advocated the use of quantum dots (QDs). QDs, which resemble the tiny semiconductors used in consumer electronics, are light-activated therapeutic nanoparticles. They can be tailored to particular infections thanks to their light-activated properties. The dots remain inactive in darkness, but can be activated on command by exposing them to light, allowing researchers to modify the light's wavelength in order to alter and kill the infected cells. The researchers have reported having achieved a 92% kill rate of drug-resistant bacteria cells in a lab-grown culture. While the superbugs can adapt and fight the therapy, the QDs can be as quickly modified so as to provide a new therapy and therefore fight back faster that this evolutionary race. The specificity of this innovation may help reduce or eliminate the potential side effects of other treatment methods, as well as provide a path forward for future development and clinical trials.
Visualization
The technique uses luminescent tags (QDs attached to proteins that penetrate cell membranes). The dots can be random in size and made of bio-inert material. They demonstrate the nanoscale property that color is size-dependent. As a result, sizes are selected so that the frequency of light used to make a group of quantum dots fluoresce is an even multiple of the frequency required to make another group incandesce. Then, both groups can be lit with a single light source.
However, the biodistribution of these nanoparticles is still imperfect due to the complex host's reactions to nano- and micro-sized materials, and the difficulty in targeting specific organs in the body. Thus, positively-charged gold nanoparticles enter the kidneys while negatively-charged gold nanoparticles remain in the liver and spleen. Even at the small size of 5 nm, these particles can become compartmentalized in the peripheral tissues and will therefore accumulate in the body over time. However, while targeting and distribution can be augmented by nanoparticles, the dangers of nanotoxicity (see the section on this topic later in this article) become an important next step in further understanding their medical uses.
Photodynamic therapy
Photodynamic therapy (PDT) involves the use of chemicals called photo sensitizers that are activated by exposure to specific wavelengths of light to release reactive molecules that can damage nearby cells. In cancer treatment, PDT damages both tumor cells and their blood supply, directly killing some tumor cells and starving those that remain of nutrients. But as with many other types of treatment, treating tumors with PDT can stimulate molecular signaling pathways that support tumor survival. The nanomedicine developed by the Wellman-based team is made up of nano liposomes - spherical lipid membrane structures enclosing a polymer nanoparticle that has been loaded with a targeted molecular therapy drug. The lipid membrane of these photo activable multi-inhibitor nano liposomes (PMILs) contains a FDA-approved photo sensitizer called BPD (benzoporphyrin derivative), and the nanoparticles are loaded with a molecular therapy drug called XL184 (or cabozantinib). XL184 inhibits two important treatment escape pathways, VEGF and MET, but while it has FDA approval to treat thyroid cancer and is being tested against pancreatic cancer and several other tumors, it is quite toxic, requiring dose restrictions or treatment interruption. Since XL184 is delivered to every part of the body and not just to the tumor when administered orally, enclosing it in the PMIL could reduce toxicity by confining its action to the area of the tumor. The more robust tumor reduction and suppression of escape pathways that is possible with PMILs might enable curative surgery or improve the outcome of chemotherapy to enhance patient survival. But while we are encouraged by these results, this combination in a new nano construct needs more clinical validation before becoming a clinical treatment option.
In PDT practice, a particle is placed within the body and is illuminated with light from the outside. The light gets absorbed by the particle and, if the particle is metal, energy from the light will heat the particle and surrounding tissue. Light may also be used to produce high energy oxygen molecules which will chemically react with and destroy most organic molecules that are next to them (like tumors). This therapy is appealing for many reasons. It does not leave a “toxic trail” of reactive molecules throughout the body (as in chemotherapy) because it is directed where only the light is shined and the particles exist. PDT has the potential for a non-invasive procedure for dealing with diseases, growth and tumors.
Skin diseases therapies
Researchers at The Hebrew University of Jerusalem (Israel) have developed a nanotechnology-based delivery system that offers a new approach to skin disease therapies. It contains a protective cellular pathway inducer that activates the body's natural defense against free radicals efficiently. This development could control a variety of skin pathologies and disorders.
The human skin is constantly exposed to various pollutants, UV rays, radiation and other stressors that exist in our day-to-day environment. When they filter into the body, they can create reactive oxygen species (ROS; these are oxygen molecules known as free radicals, which are able to damage and destroy cells, including lipids, proteins and DNA). In the skin (the largest organ of the body), an excess of ROS can lead to various skin conditions, including inflammatory diseases, pigmenting disorders, wrinkles, and some types of skin cancer; they can also affect internal organs. This damage is known as oxidative stress (OS). The body is naturally equipped with defense mechanisms to counter oxidative stress. It has anti-oxidants and, more importantly, anti-oxidant enzymes that attack the ROS before they cause damage.
In collaboration with her colleagues at The Technion (Israel Institute of Technology), Maya Ben-Yehuda Greenwald.22 of the The Hebrew University of Jerusalem suggested an innovative approach that uses the body's own defense system to invigorate it to produce antioxidant enzymes, while maintaining skin cell redox balance – a gentle equilibrium between ROS and their detoxification. Production of antioxidant enzymes in the body is signaled in the DNA by activation of Nrf2 a powerful protein that exists in every cell in our body. This Nrf2 cellular-protective signaling pathway is a major intersection of many other signaling pathways affecting each other and determining cell functionality and fate. Nrf2 is capable of coordinating the cellular response to internal as well as external stressors by tight regulation of phase-II protective enzymes, such as the antioxidant enzymes. The approach employs a unique delivery system that may leverage dermal cure. The research team showed for the first time that applying nano-size droplets of micro emulsion liquids containing a cellular protective pathway inducer into the skin activates the natural skin defense systems.22 Ben-Yehuda Greenwald has also discovered a new family of compounds capable of activating the Nrf2 pathway. Moreover, by incorporating them into the unique delivery system she has developed, she managed to efficiently stimulate the activation of the Nrf2 pathway and mimic the activity of the body's natural way of coping with a variety of stress conditions. The formula she has created could be used in topical medications for treating skin conditions. It could also be used both as preventive means and for treatment of various skin conditions, such as infections, over-exposure to UV irradiation, inflammatory conditions, and also internal disease (Figure 3).

Figure 3 Consequences of Skin Exposure to Stressors.23
While the researchers focused on the skin, the formulation could prove to be effective in enhancing the body's natural protection against the damaging effects of ROS in other parts of the body, such as inflammation in cardiovascular diseases, heart attack, cancer, multiple sclerosis and Alzheimer's.
Still other applications include
New approaches to developing highly potent Nanomedicine drugs
Biologically efficient inhibition of disease-causing organisms
A new study led by University of Kentucky researchers [23] suggests a new approach to developing highly-potent drugs which could overcome current shortcomings of low drug efficacy and multi-drug resistance in the treatment of cancer as well as viral and bacterial infections. Inhibiting multi-subunit targets works similar to the series-circuit Christmas ecorating light chains: one broken bulb turns off the entire lighting system. The study has identified a new mechanism of targeting multi-subunit complexes that are critical to the function of viruses, bacteria or cancer, thus reducing or possibly even eliminating their resistance to targeted drugs. The same research group has also been active in the use of ribonucleic acid (RNA) nanoparticles and a viral nano-motor to fight cancer, viral infections and genetic diseases, and constructing RNA nanoparticles as drug carriers.
By targeting RNA or protein subunits that have multiple sites for inactivation, but that are inextricably linked, this method allows for killing or disabling the RNA or protein without requiring the inhibition of multiple pathways that might be used by the organism to remain active and viable. Thus, multiple drugs are not needed as well. Using this method - a single subunit targeting to the target RNA or protein subunits that are unique and assenting for the organism - the organism will be disabled or die and thus no longer be able to cause disease.
While one of the vexing problems in the development of drugs is drug resistance, the above study has identified a new mechanism of efficiently inhibiting biological processes that are critical to the function of the disease-causing organism, such that resistance is minimized or eliminated.
Healing of burn wounds
Skin thermal burns are a complex and major source of morbidity, mortality and healthcare expenditure in the United States, with 486,000 patients treated for burn injury each year. Fire, flame and scald burns account for roughly 77% of burns. Given the range of causes, from fire associated injury to water scalding, patients often present with multiple and complex burn wounds that often worsen and expand over the first few days due to the associated underlying inflammation and injury. Furthermore, burns are dynamic injuries that undergo expansion within the first 24-72 hours with histological evidence of both apoptosis and necrosis in the surrounding tissue. Though the impact of these injuries is large, our current treatment armament falls short with gold standard treatments lacking evidence to support their use, or even worse, may even delay wound healing as was recently elucidated with silver sulfadiazine.
Recent investigations have turned to the study of endogenous molecules as treatments for a variety of ailments including burns. One such molecule of interest is nitric oxide (NO), given its complex and necessary role in the wound healing process. However, use of nitric oxide is limited due its exceedingly short half-life. In order to overcome these limitations, investigators have turned toward the use of nitrosating substances (those that donate NO molecules) in order to harness NO’s wound healing potential. Nitric oxide (NO) and NO-donating nitrosothiols (RSNOs) are key orchestrators of all three phases of wound healing, both having a broad and sometimes distinct array of roles. Nitrosating substances such as RSNOs have been proven to promote wound closure due to their ability to modulate inflammation, cytokine production and vascular function.
A unique nanotechnology has been used that can both release the potent bio molecule nitric oxide (NO) over time, as well as facilitate nitrosation (the addition of an NO group to a biological molecule, which is central to many of NO’s activity). It consists of N-aceylcysteine S-nitrosothiol (NAC-SNO) nanoparticles that can prevent wound expansion and accelerate wound closure in a murine burn model. The role of nitric oxide in wound healing is well established through all three phases. Both NO itself and the act of nitrosation are exceedingly important in the transition from the inflammatory phase to the proliferative phase of wound healing. NAC-SNO nanoparticles clinically accelerate burn wound healing. While still in its infancy, this technology will be brought from bench to bedside as it has been licensed by the company Nano Biomed Inc.
The above model is adapted from previous studies, and allows for the evaluation of multiple burns, their expansion, and the impact on surrounding tissue. Several previously described properties of the nanotechnology platform such as increased capacity to generate NO, enhanced vasodilatory effect, and low toxicity make it an attractive candidate as a potential therapeutic adjuvant.
Nanomedicine and immunosystem impairment through oxidative stress
Oxidative stress is occurring selectively even at low levels of exposure to nanoparticles. Scientists at the U.S. Department of Energy's Pacific Northwest Laboratory .24 have shown that this process is at work during encounters between certain nanoparticles and immune cells, selectively modifying proteins on macrophages (a type of immune cells) (Figure 4). The idea that a nanoparticle would damage the body's macrophages is no surprise: Macrophages are the body's first responders when it comes to recognizing and neutralizing an invader. Some nanoparticles can weaken macrophages' ability to recognize, hold onto, and engulf the particles. While oxidative stress is a common way for cell damage to occur, the above finding was a surprise in some ways. The approach developed is sensitive enough to detect effects of nanoparticles on macrophages long before those cells die. This gives the opportunity to understand the most sensitive cellular targets of oxidative stress and the pathways involved more completely than before. This is important information for understanding how nanoparticles can alter cell function and for beginning to identify functions that allow cells to adapt versus those that are potentially involved in adverse effects.
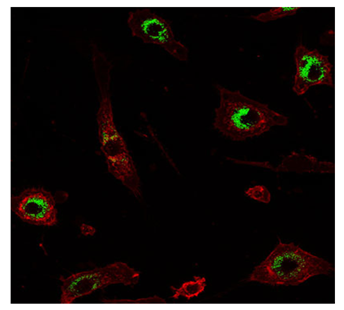
Figure 4 Mouse macrophages (red) engulf silica nanoparticles (green) 25.
The above method, known as a “quantitative redox proteomics” (QRP), utilizes an advanced mass spectrometer to look at thousands of sites involved in redox reactions simultaneously. It is a very sensitive measure of protein modifications in cells to allow scientists to look at specific sites in the cell where certain functions are known to be carried out. In an earlier study, the researchers found that when macrophages are exposed to nanoparticles, the cells do not function as well and are less able to recognize and remove Streptococcus pneumonia, the leading cause of community-acquired pneumonia. The pattern of protein changes identified in this study provides new clues to the types of nanoparticles that cause these effects and the proteins involved.
Infusion-related reactions in nanomedicine
The human body's first line of defense against blood-borne intruders (the”complement system”) is blamed for infusion-related reactions to nanomedicines, but the conventional models used to predict the risk of cardiopulmonary side effects in response to nano pharmaceuticals might not well represent what actually occurs in humans. This assertion has been advanced by Moghimi.25 of the University of Copenhagen, Denmark.25 who questioned the validity of pig and sheep models to predict the risk of infusion-related reactions to nanoparticle-based medicines in humans.
Moghimi proposed that some individuals may be highly sensitive to nanoparticles due to a particular liver or lung disorder or a predisposition to liver or lung disease. Future studies should compare human lung tissue from patients with and without liver and inflammatory lung disease to explore the role of the complement system in nano pharmaceutical related infusion reactions. In addition, he also suggested that a more realistic and predictive model for examining the risk of cardiopulmonary side effects associated with nanomedicines may be a rat with cirrhosis of the liver.
Note on nanotechnology development and nanomedicine
Nanotechnology is being applied to almost every field imaginable, including electronics, magnetic, optics, information technology, materials development, and biomedicine. The development of truly revolutionary nanotechnology products, materials and applications, such as nano robotics, are years in the future (some say only a few years; some say many years). What qualifies as "nanotechnology" today is basic research and development that is happening in laboratories all over the world. "Nanotechnology" products that are on the market today are mostly gradually improved products (using evolutionary nanotechnology) where some form of nanotechnology enabled material (such as carbon nanotubes, nano composite structures or nanoparticles of a particular substance) or nanotechnology process (e.g. nano patterning or quantum dots for medical imaging) is used in the manufacturing process. In their ongoing quest to improve existing products by creating smaller components and better performance materials, all at a lower cost, the number of companies that will manufacture nano products (by this definition) will grow very fast and soon make up the majority of all companies across many industries. Evolutionary nanotechnology should therefore be viewed as a process that gradually will affect most companies and industries. This foretells enhanced developments and applications in nanomedicine.
The development of truly revolutionary nanotechnology products, materials and applications, such as nano robotics, are years in the future. What qualifies as "nanotechnology" today is basic research and development that is happening in laboratories all over the world. "Nanotechnology" products that are on the market today are mostly gradually improved products where some form of nanotechnology-enabled material or nanotechnology process is used in the manufacturing process. Nanomedicine refers to highly specific medical interventions at the molecular scale for curing disease or repairing damaged tissues, such as bone, muscle, or nerve. Although products classified as nanomedicine products have indeed appeared over the past decade, such products have not exactly revolutionized treatment paradigms as envisaged earlier.
Nanomaterials represent a new class of materials, where ever-smaller length scales (<100 nm) impart altered or enhanced properties. Three challenges in this area are: Control of the structure of nanomaterials, control and definition of the spatial placement of such materials, and toxicity of the resulting structures (particularly in the case of medical and health care applications). Nano devices are valuable aides in the delivery life-saving drug treatments (cancer, neurological diseases, etc.) at the cancer sites. Such minute devices have the potential to be engineered to efficiently and more safely deliver drug treatments directly to the location of diseased cells while helping avoid harm to healthy cells that fall victim to toxic drugs administered by conventional means.
However, nanoparticles are not free of risks or adverse reactions. The major obstacles to be overcome include more stringent safeguards for handling nanoparticles and their perceived or real toxic effects. Further, there are infusion-related reactions to nanomedicines. Thus, oxidative stress is occurring selectively even at low levels of exposure to nanoparticles. This has been observed where nanoparticles damage the body's macrophages (the body's first responders when it comes to recognizing and neutralizing an invader). Thus, some nanoparticles can weaken macrophages' ability to recognize, hold onto, and engulf the particles. When macrophages are exposed to nanoparticles, the cells do not function as well and are less able to recognize and remove Streptococcus pneumonia, the leading cause of community-acquired pneumonia. The pattern of protein changes identified provides new clues to the types of nanoparticles that cause these effects and the proteins involved.
After providing a definition and a brief history of nanomedicine, I have outlined and classified its numerous fields of application. We then described medical uses of nanomaterials and nano devices: in vivo imaging; delivery of a much higher dose of medication using (a) platelet-coated nanoparticles and the correspondingly associated numerous advantages (circulation throughout the bloodstream without being attacked by the immune system; preferential binding to damaged blood vessels and certain pathogens; non-toxicity as the platelet membranes are made of a biodegradable polymer that can be safely metabolized by the body; packaging with many small drug molecules that diffuse out of the polymer core and through the platelet membrane onto their targets; treatment focus on the bacteria locally without spreading drugs to healthy tissues and organs throughout the rest of the body; and, when disguised as red blood cells, capability of removing dangerous pore-forming toxins; (b) shape-shifting engineered nanoparticles that can target cancer cells, expose a drug molecule to the cancerous cell, and tag the cancerous cells with a signal molecule; (c) While efforts to apply nanotechnology in cancer have focused almost exclusively on the delivery of cytotoxic drugs to improve therapeutic index, molecularly targeted agents, in particular kinase inhibitors, can also present considerable therapeutic index limitations; and (d) development of disease biomarkers in the form of nucleic acids, the building blocks of all living organisms potentially a first-line, non-invasive diagnostic to detect anything from cancer to the Ebola virus.
I have surveyed the use of nanoparticles in several diagnoses and treatments of cancer: (a) imaging and eradication (because nanoparticles have high surface area to volume ratio, many functional groups could be attached to a nanoparticle to seek and bind to certain tumor cells. It is even possible to manufacture multifunctional nanoparticles that would detect, image, and then proceed to treat a tumor); (b) virtual biopsy and treatment of brain metastatic tumors employing a qualitative/quantitative “MRI virtual biopsy” method based on a nanoconjugate-carrying MRI contrast agent gadolinium-DOTA and antibodies recognizing tumor-specific markers and extravasating through the blood brain barrier; (c) lung cancer and chronic obstructive pulmonary disease as nanoparticles can penetrate deep into lung compartments that are otherwise impossible to reach; (d) pancreatic cancer using nanoparticles that combine photodynamic and molecular therapies in a nanoparticle drug-delivery system that combines two complementary types of anticancer treatment to improve patients' outcomes; and (e) ocular cancer in which a new nanoparticle uses the tumor cell’s protective mechanism against itself, short-circuiting tumor cell metabolism and killing tumor cells.
The applicability of nano treatment to cardiovascular diseases (arteriosclerosis) is based on the restoration of the original condition of healthy endothelial cells by guiding replacement cells to diseased vascular segments using nanoparticles using a combination of nanotechnology with gene and cell therapy together with custom-made magnetic fields to enable the circumferential re-endothelialization of vessels and improve the vascular function.
There are numerous other applications such as in: (a) Surgery by the development of a flesh welder to weld perfectly arterial walls using gold-coated nanoshells dribbled along the surgical seam; (b) chemotherapy using nanotechnology to light up common cancer drugs to be able to see where the chemotherapeutic drug goes and how long it takes to travel to the site of interest. This is in opposition to organic dyes (that fade quickly) and toxic elements (particularly, metals); and (c) treatment of antibiotic-resistant superbugs employing quantum dots tailored to particular infections thanks to their light-activated properties.
Still other applications include: (a) Visualization with quantum dots attached to proteins that penetrate cell membranes. However, the biodistribution of these nanoparticles is still imperfect, due to the complex host's reactions to nano- and micro-sized materials, and the difficulty in targeting specific organs in the body; and (b) photodynamic therapy in which a particle is, placed within the body, when illuminated from the outside, absorbs the energy from the light to heat the particle and surrounding tissue. Alternatively, light is used to produce high-energy oxygen molecules which will chemically react with and destroy most organic molecules that are next to them (like tumors).
Lastly, I have summarized novel approaches to develop highly potent drugs including: (a) Developing highly-potent drugs which could overcome current shortcomings of low drug efficacy and multi-drug resistance in the treatment of cancer as well as viral and bacterial infections. This is a new mechanism of targeting multi-subunit complexes that are critical to the function of viruses, bacteria or cancer, thus reducing or possibly even eliminating their resistance to targeted drugs. While one of the vexing problems in the development of drugs is drug resistance, this approach has identified a new mechanism of efficiently inhibiting biological processes that are critical to the function of the disease-causing organism, such that resistance is minimized or eliminated; and (b) In the healing of burn wounds, nitrosating substances that donate nitrous oxide have been proven to promote wound closure due to their ability to modulate inflammation, cytokine production and vascular function. A unique nanotechnology has been used that can both release the potent bio molecule nitric oxide over time, as well as facilitate nitrosation (the addition of a nitrous oxide group to a biological molecule) so as to prevent wound expansion and accelerate wound closure.
None.
None.

©2016 Fymat. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.