Journal of
eISSN: 2377-4282


Opinion Volume 4 Issue 4
Department of Bioengineering, Izmir Institute of Technology, Turkey
Correspondence: Ahu Arslan Yildiz, Department of Bioengineering, Izmir Institute of Technology, Izmir, 35430, Turkey
Received: December 01, 2016 | Published: December 9, 2016
Citation: Demircak N, Arslan-Yildiz A (2016) On-Chip Drug Screening and Nanomedicine Applications via (L)SPR. J Nanomed Res 4(4): 00094. DOI: 10.15406/jnmr.2016.04.00094
Microfluidic lab-on-a-chip tools have been emerged in recent years as next generation nanomedicine and drug screening platforms. Plasmonic microfluidic systems have shown promising progress over the past years to identify potential drug candidates. These systems allow the study of drug interactions at molecular and cellular level while facilitating fast, sensitive and label-free detection. We foresee that the advancements in Surface Plasmon Resonance (SPR) coupled microfluidic systems will be an easy to use and cost-effective alternative to identify potential drug candidates for drug development process.
Keywords: Lab-on-a-chip, Drug screening, Microfluidics, Surface plasmon resonance, Localized surface plasmon resonance, Nanomedicine
Drug screening is the first phase of a drug development process taking 10 to 15 years for detecting potential drug candidates. Screening of candidates on drug targets is a random process and functions of drugs are not known in most trials, therefore traditional drug screening is not efficient and expedite process. In vitro nanomedicine and drug screening platforms provide significant contribution to thescreening approach byaccelerating the overall process prior to in vivo animal and clinical experiments.1,2 Microfluidic systems and microdevices have been utilized in recent years for drug screening analysis since they can be easily adapted to in vitro biological systems and bioanalitical tools. Advancements in miniaturized devices, such as microfluidic lab-on-a-chip tools have significantly increased the accuracy and cost-effectiveness of sample analysis in pharmaceutical field. In addition, these technologies show promise for pharmaceutical and drug discovery field owing to cost-effective, rapid , portable, low sample volume and high-throughput analysis features.3,4
Engineering microfluidic (l)spr platforms
Newly developed microfluidic drug screening platforms employing plasmonic sensing strategies enables detecting specific drug-target interactions at molecular level.5 Metal (generally Au and Ag) nanoparticle/film based plasmonic sensors such as surface plasmon resonance (SPR), localized surface plasmon resonance (LSPR) have been integrated into microfluidic systems for efficient drug screening analysis (Figure 1A).6-7 There are several advantages of these plasmonic assays such as high sensitivity and selectivity, label-free and real-time detection, high-throughtput analysis.6
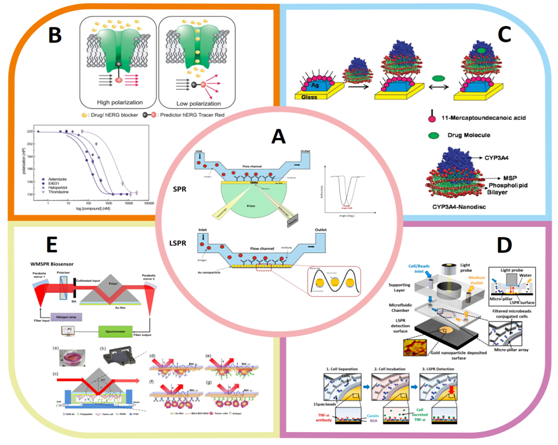
Figure 1 On-Chip Drug Screening and Nanomedicine Applications Via (L)SPR.
Schematic illustration of (A) SPR and LSPR sensor, (B) hERG integrated biomimetic membrane platform and dose response curves, Reprinted by copyright permissions from.5 (C) CYP3A4-Nanodisc immobilized Ag nanobiosensor and drug interaction, Reprinted by copyright permissions from.8 (D) LSPR optofluidic platform device, Reprinted by copyright permissions from.9 (E) Wavelength-modulated surface plasmon resonance (WMSPR) biosensor and representation of cell sensing on Au film, Reprinted by copyright permissions from.10
To achieve the effective and improved screening levels, Jie He and colleagues have utilized a novel microfabrication methodology integrating LSPR into microfluidic lab-on-a-chip device. Photolithograpy and hole mask colloidal lithograpy techniques were employed in combination to fabricate microfluidic device forming patterned, uniform nanoparticle arrays for investigation of protein binding kinetics. This approach facilitates rapid design and fabrication process and also improves efficiency of biomolecule screening. Therefore it has been suggested for further analysis such as drug screening, genetic screening and bioassays.11
Drug screening at molecular and cellular level
In vitro drug screening tests have been carried out at molecular (supported lipid bilayer etc.) and cellular levels by using (L)SPR- coupled microfluidic platforms.5,12,13 Indeed, there have been tremendous effort to develop such tools and models to show capabilities and limitations for drug screening applications. For example, we have demonstrated the interaction of drug molecules and hERG (human ether-a-go-go-related gene) potassium channel on artificial lipid membrane platform containing hERG channel (Figure 1B).5 SPR coupled microfluidic chip was utilized for in vitro expression and insertion of hERG ion channel into supported lipid bilayer through cell-free expression technique. Drug and hERG interaction was validated via imaging SPR (iSPR) and Surface Plasmon Enhanced Fluorescence Resonance Spectroscopy (SPFS). Developed artificial model membrane presents an alternative experimental platform for high-throughput screening because of robust structure and rapid response. In another example, soluble nanometer scale membrane bilayer disks (Nanodisk) were formed on Ag nanoparticle surfaces (Figure 1C). To create a screening platform for different drug candidates Cytochrome P450s(CYP3A4) enzyme was stabilized using Nanodisk and evaluated by LSPR.8
In addition to measuring binding kinetics of drugs at the molecular level, (L)SPR techniques have also beenused to analyze cell morphology alteration, cell-protein interaction, and other biological interactions at cellular level for drug screening and nanomedicine applications.10,12,14 While building a realistic cellular model, it is important to engage a microfluidic on-chip platform which facilitates mimicking the real tissue and organ response in a small scale. For example, Wang and colleagues demonstrated drug resistance of herceptine (a monoclonal antibody drug for breast cancer) by imaging SPR technique. Binding kinetic of Herceptine to Her2 which is the specific target of herceptine, was examined by utilizing intact cell-based binding assay. On-chip breast cancer cells (SK-BR3 and BT474) and human epithelial carcinoma cells (MCF7 and HeLa) were analyzed through alterations of SPR signal intensity depending on varied drug concentration.15
More interestingly, immune system and tumor models have been developed which represents the real responses in vivo. A pioneering example, Oh and colleagues have created a microfluidic LSPR immunoassay system to detect cytokines secreted by immune system cells in human blood. The system consists of microfluidic and support layer on top of Au nanoparticle decorated LSPR sensing layer (Figure 1D).9 Yimin Wang et al. developed a wavelength-modulated surface plasmon resonance (WMSPR) sensor chip that measure expression level of biomarkers on the surface of tumor cells as quantitative and real time analysis (Figure 1E).10 Although above mentioned microdevices have been suggested for diagnosis purposes, it can be easily adapted to drug screening approaches to analyze effects of drug candidates. We anticipate that with further advancements on (L)SPR-coupled microfluidic chip technologies, developed tools can be further associated with drug screening and nanomedicine applications instead of conventional techniques.
Nanomedicine and drug development fields have made dramatic advancements to enable rapid, label-free, sensitive and selective, real time analysis of drug candidates. Many other studies including our own work are focusing on the progress of microfluidic lab-on-a-chip technologies towards the final aim of constructing a viable and accurate drug screening platform. There are enormous efforts in development of (L)SPR-coupled microfluidic technologies especially for drug development and screening which promotes the nanomedicine research at the molecular and cellular level. Overall, the use of (L)SPR-coupled microfluidic technologies in drug screening and nanomedicine applications provide new opportunities and directions to expand the knowledge in these fields, as well advance the next generation tools for drug development process for clinical translation.
None.
None.

©2016 Demircak, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.