Journal of
eISSN: 2377-4282


Research Article Volume 1 Issue 2
1Centre of Advanced Study in Marine Biology, Annamalai University, India
2Department of Molecular Biology and Genetics, Istanbul Medeniyet University, Turkey
Correspondence: Satyavani K, Department of Molecular Biology and Genetics, India, Tel 91 9789585377
Received: November 02, 2014 | Published: November 27, 2014
Citation: Satyavani K, Gurudeeban S, Ramanathan T (2014) Influence of Leaf Broth Concentration of Excoecaria Agallocha as a Process Variable in Silver Nanoparticles Synthesis. J Nanomed Res 1(2): 00011. DOI: 10.15406/jnmr.2014.01.00011
A simple, cost effective and time consuming approach for biosynthesis of monodispersive silver nanoparticles (AgNPs) by Excoecaria agallocha leaf extract and its free radical scavenging activity is described. Different concentration of E. agallocha leaf broth solution (EALB) is used as a process variable to optimize nanoparticles synthesis with 0.1mM silver nitrate (AgNO3) at a 1:4 ratio. Nanoparticles was characterized by observation of colour changes, Ultraviolet spectroscopy (UV Spec), Transmission Electron microscopy (TEM) and Fourier transform infra red spectroscopy (FT-IR). We observed 97% of synthesized AgNPs with dark purple colour and mono dispersive spherical shaped 15-45nm at 25% concentration of EALB at 330nm absorbance within the period of 10min incubation than compared to others. FT-IR spectra indicated the functional group of protein and polyphenols acts as a capping agent on the surface of silver nanoparticles and gave proper stabilization. This is the first report on eco-friendly green synthesis approaches for silver nanoparticles synthesis from ancient Thillai tree. 40µg of optimized silver nanoparticles exhibited significant DPPH free radical scavenging activity. Further studied will be needed to find out the exact mechanism of action of silver nanoparticles.
Keywords:Absorption, Dark purple, Free radical, Polyphenols, Spherical
gNPs, Silver NanoParticles; EALB, Leaf Broth solution; UV Spec, Ultraviolet spectroscopy; TEM, Transmission Electron microscopy; FT-IR, Fourier Transform Infra Red spectroscopy
Interest towards the use of nanoscale particles is rising due to their specific size, shape and distribution. Especially, surface area of the nanoparticles increased automatically the biological activity is increased. Colloidal silver particles have found tremendous applications in the field of high sensitivity bimolecular detection, diagnostics and antimicrobials.1,2 In general, the nanoparticles are produced by different physical, photochemical reactions in reverse micelles, radiation assisted and chemical methods.3 But in the above cases, wide range of energy, solvents and toxic chemicals cannot prevent that. In the 20th century, researchers focused their more interest towards biological material such as plants, Calli tissues, microorganisms and enzymes4,5 for nanoparticles synthesis. Then compared to other sources, using plant for nanoparticles synthesis is highly advantageous to avoid the long term biomass culture and maintenance.
Excoecaria agallocha is widely distributed medicinal mangrove among the ancient Thillai Natarajar temple and Pichavaram wetlands in Tamil Nadu. It has traditionally been applied to treat sores, ulcers, and smoke from the bark to treat leprosy. Previous phytochemical investigation studies of E. agallocha revealed that the presence of diterpenoids, triterpenoids, flavonoids and phorbole esters.6 Leaf extract of E. agallocha used for rheumatism, paralysis, cutaneous infection and abortificant. Several preclinical and clinical trials showed its potential as anti-HIV, anticancer, antibacterial, antiviral, antidiabetic and diabetic foot ulcer treatment.7,8 Free radicals are in charge of causing oxidative stress. Antioxidants play a vital role in free radical scavenging and prevent various diseases. Due to modern life style people don’t have time to manage our self as a result numerous disease and disorders are rising casually. The study of free radical scavenging activity of these noble biosynthesized nanoparticles such as silver gives an idea about its association with biomolecules of living systems.9` Therefore, the present study aimed to investigate different concentration of E. agallocha leaf broth in the reduction mechanism of silver ions into silver nanoparticles, characterize and determine its free radical scavenging activity using DDPH assay.
Collection and Preparation of Excoecaria agallocha leaf broth
Fresh leaves of E. agallocha were collected during December 2012 in the Kodiyampalayam coastal village, located in Nagapattinam district, southeast coast of India (Figure 1). The plant was identified with the specimen deposited in the herbarium of Centre of Advanced Study in Marine Biology (AUCASMB 63/2010), Faculty of Marine Sciences, Annamalai University, Parangipettai, Tamil Nadu, India. Silver nitrate used for biosynthesis was purchased from Sigma-Aldrich (India). The plant was washed with distilled water and finely cut in to small pieces. A known amount of leaves was added to 100 mL of deionized water and boiled for 15 min in a water bath. The mixture was filtered with Whatmann No.1 filter paper to obtain aqueous extract of definite concentrations.
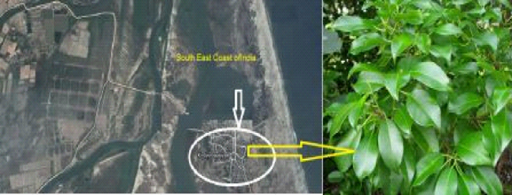
Figure 1 Study area - Kodiyampalayam coastal village, Nagapattinam District, Southeast coast of India and study plant Excoecaria agallocha.
Influence of leaf broth concentration on silver nanoparticles synthesis
In this study, we used different leaf broth concentration as a process variable to study the silver nanoparticles synthesis with constant 0.1mM AgNO3 solution at a 1:4 ratio. Briefly, 5, 10, 15, 20, 25 and 30% of different concentrations of E. agallocha leaf broth (EALB) used to determine its potential on silver nanoparticles (AgNps) synthesis. 0.1 mM AgNO3 was added into different concentration of EALB at a 1:4 ratio. The reaction mixture was incubated at room temperature under shaking condition at 200×g for the reduction of silver ions into nanoparticles and observed the colour changes.10 After that, the reaction mixture contains synthesized silver nanoparticles was centrifuged at 10,000×g for 15min and the pellet was rinsed with distilled water to get rid of unwanted biological molecules. The same process was repeated thrice and the freeze dried samples were used for the further analysis.
UV spectroscopy analysis
The rate of nanoparticles synthesis was confirmed by UV spectrophotometer (UV- 2450(Shimadzu) absorbance between 200 and 800nm.
Transmission Electron Microscopy (TEM)
Bright-field transmission electronic images were taken using (JEOL JEM 2100) TEM. Japan, operated at 200 keV. Purified samples were stained with 2% (w/v) phosphotungstic acid for 30S and placed on copper grids with films for viewing. Samples for TEM measurements were prepared by dropping the dispersion of the nanoparticles on a copper grid supported Form var Films.
Fourier Transform Infra-Red Spectroscopy (FT-IR)
To identify the silver nanoparticles associated functional groups by Avatar 660 FT-IR spectroscopy (Nicolet, USA) using KBr pellets. To obtain a good signal to noise ratio, 256 scans of silver nanoparticles were taken in the range of 400-4000cm-1 and the resolution was kept at 4 cm-1
Free radical scavenging activity of optimized silver nanoparticles
2,2-diphenyl-1-picrylhydrazyl is commonly used assay for determination of free radical scavenging activity. Briefly, the optimized 25% of EALB only utilized as a control. Similarly different concentrations of optimized silver nanoparticles and standard (10, 20, 30, 40 and 50μg mL-1) were prepared and used for this assay. Reaction mixture was prepared by adding 100μL of AgNPs having a range of concentration into 100μL DPPH solution. The reaction mixture was incubated for 30min at room temperature under shaking conditions. Read the absorbance at 517nm in 96 well plates. Ascorbic acid is used as a standard. All the measurements were carried out after 30min and mixing the insoluble nanoparticles with DPPH reagents. Scavenging activity was expressed as a percentage of inhibition. Experiments were made in triplicate.
Influence of leaf broth in synthesis of silver nanoparticles
In this study, silver nanoparticles were successfully synthesized using EALB as a process variable. The light reddish brown, dark brown and dark purple colour of synthesized nanoparticles was observed within 10min different concentration of EALB with 0.1mM AgNO3 at 1:4 ratios (Figure 2). The colour was darker with increased incubation time. After 25min there was no significant colour change in the reaction mixture. It indicated the completion of reduction reaction, than compared to other EALB, the 25% with aqueous silver nitrate showed a 97% reduction within 10min.
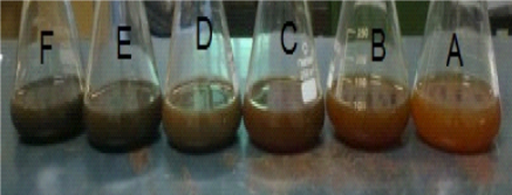
Figure 2 Biosynthesis of Silver nanoparticles by different concentration of E.agallocha Leaf broth with ion (5, 10, 15, 20, 25 and 30%) with 0.1 mM AgNO3 solution at 1:4 ratios within 10 minutes.
UV-Spectroscopy analysis
UV spectra of synthesized AgNO3 obtained from different leaf broth concentration (5, 10, 15, 20, 25 and 30%) were shown in (Figure 3). The UV absorption spectra showed both strong and weak resonance bands. The absorption spectrum is indirectly proportional to the leaf broth concentration. 470, 450, 440, 445, 330 and 340nm were observed in 5,10,20,20 and 30% of EALB with 0.1mM of AgNO3. Then compared to others strong resonance band was observed in 25% of EALB.
Morphology analysis by TEM
In the present study, the TEM micrograph of synthesized silver nanoparticles with respective scale bar and size were showed in (Figure 4a-4f). We observed spherical and few non spherical silver nanoparticles. The concentration of leaf broth is directly proportional to nano meter size of the particles. Also some aggregation was showed in (Figure 4a-c) it due to less concentration of leaf broth and its functional groups involved in stabilization of biologically synthesized nanoparticles. Then compared to others, 25% of EALB with 0.1mM of AgNO3 biosynthesized silver nanoparticles was monodispersive, spherical shape with 15 to 45nm in size. The average particle size obtained from E. agallocha was 22 nm calculated by Standard deviation. Based on the color, UV absorption and TEM analysis, the optimized biosynthesized silver nanoparticles (25 % of EALB with 0.1mM of AgNO3) was used for FT-IR analysis.
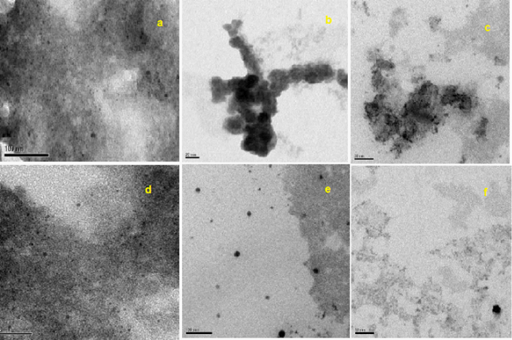
Figure 4 Transmission electron micrograph of synthesized silver nanoparticles with respective scale bar and size.
a) 5% of leaf broth with 0.1M AgNO3 showed 20 -75 nm
b) 10% of leaf broth with 0.1M AgNO3) showed 30 to 75 nm
c) 15% of leaf broth with 0.1M AgNO3) showed 30 to 65 nm
d) 20% of leaf broth with 0.1M AgNO3) showed 35 to 55 nm
e) 25% of leaf broth with 0.1M AgNO3) showed 15-45 nm
f) 30% of leaf broth with 0.1M AgNO3) showed 20-45 nm
FT-IR analysis
The infra red absorption spectrum of 25% EALB and synthesized silver nanoparticles (25%EALB with 0.1mM AgNO3) were showed in (Figure 5a & 5b). Both of them showed some characteristic same peak. The absorption spectra of synthesized silver nanoparticles showed additional peaks it represents the functional group involved in the nanoparticles synthesis using silver nitrate. Such as the absorption peak at 2926.01 and 2864.29 cm-1 can be assigned as CH stretches, PH stretch at 2353.16cm-1, stretch vibration of –C=C- is assigned to the amide 1 bonds of proteins at 1633.71 cm-1, CH3 in aliphatic compounds corresponds at 1440.83 cm-1,1388.75 correspond to the amide III and II group respectively, peak at 663.51 cm-1 which represents the aromatic ring C-H vibrations, indicate the involvement of free catechin,1051.20 cm-1 corresponds to C-N stretching vibrations of aliphatic amines or to alcohols or phenols representing the presence of polyphenols and indicate C-O single bond at 1101.35. Thus it is clear that these peaks were observed due to reduction of silver ions by 25% of EALB with 0.1mM AgNO3. It might be due to the presence of protein and poly phenolic group in this leaf broth extracts. These are acted as stabilizing and capping agent for nanoparticles synthesis.
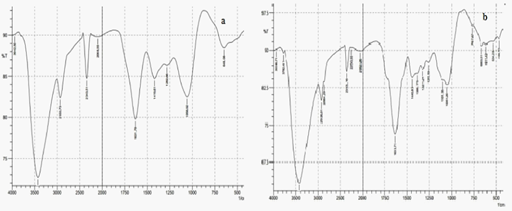
Figure 5 FT-IR absorption spectra. a) Characteristic peak of 25% of EALB b) Characteristic peak of synthesized silver nanoparticles (25% of leaf broth with 0.1M AgNO3).
Free radical scavenging activity of nanoparticles
The free radical scavenging potential of optimized silver nanoparticles was showed in (Table 1). Among these, 40μgmL-1 showed significant DPPH radical scavenging in a dose dependent manner (82.17%) than compared to standard.
Concentration (μg mL-1) |
% of Free radical scavenging |
|
Silver Nanoparticles |
Ascorbic acid |
|
10 |
22.34% |
36.44% |
20 |
43.13% |
51.22% |
30 |
67.43% |
70.33% |
40 |
82.71% |
87.55% |
50 |
78.53% |
88.12% |
Table 1 Free radical scavenging activity of various concentrations of optimized silver nanoparticles (25% EALB with 0.1mM of AgNO3)
Use of biologically synthesized nanoparticles increased every year due to various biomedical applications.11 In this view, silver nanoparticles were synthesized using different leaf broth concentration of E. agallocha and evaluated its free radical scavenging activity. The synthesized nanoparticles showed dark brown and purple colour due to the excitation of surface plasmon resonance of synthesized nanoparticles. Similar results were observed in Heliotropium curassavicum with 20% concentration of leaf broth within the period of 15min incubation.10 The preliminary detection of AgNPs was carried out by visual observation of the color changes of the reaction solutions and confirmed clearly by UV spectrum. Previously, Link & Ei sayed12 mentioned that the absorption spectroscopy is a very useful technique for the characterization of nanoparticles. Especially, the observation of strong broad surface Plasmon peak has been well observed in metal nanoparticles with size range of 5 to 100nm. The Maxwell equation reported that the both conduction and valence band in metallic nanoparticles lies very close to each other, in this case the electron moves freely. These free electrons give rise to a SPR absorption band due to the collective oscillation of nanoparticles electrons with the light wave.13 Compared to others, 25 % of EALB with 0.1mM of AgNO3 showed band at 330 nm with 15 to 45nm spherical size nanoparticles which is directly proportional to the SPR peak shift towards shorter wave length.14 It attributed to the excitation of surface plasmon resonance (SPR) in the metal nanoparticles.15 Similar size of silver nanoparticles was obtained from Rhododendron dauricum.16 In the green synthesis bioprocess, inherent capping and stabilizing provide an additional advantage. Plants primary and secondary metabolites played a vital role in the stabilizing process against coagulation. The lesser concentration of 5, 10 and 15% of E.agallocha showed some clumps from TEM. In accordance with Mulvaney,17 the synthesized metal nanoparticles must be stabilized against van der Waal force because this force might be involved in coagulation process. We observed monodispersive spherical shape silver nanoparticles from 25% of EALB with 0.1mM AgNO3. But we observed some aggregation which might be due to insufficient of plant metabolites in the capping of synthesized nanoparticles. Bar et al.18 depicted, the molar concentration of silver nitrate solution decreased, the particle size also decreased. In the present study, 0.1mM AgNO3 used for silver nanoparticles synthesis and the average size of biosynthesized silver nanoparticles not exceed more than 75nm. In the present study protein and polyphenols might be responsible for the stabilization, which creates steric or electro steric barriers around the nanoparticle surface. Under careful observations, it is evident that the silver nanoparticles surrounded by a faint thin layer of other material, it could be due to the capping of phytoconstituent from E. agallocha which is clearly evidenced by FT-IR verdicts. Whenever there is an increase in the leaf extract concentration in silver nitrate mixture it decreased the nanoparticles size.19 We observed slight size 20nm size particles using 25% of EALB. Plant metabolites have the capacity to reduce the oxidation damage caused by free radicals. Number coastal medicinal plant extracts and bio-fractions reported potential free radical scavenging activity.20 Interactions between the optimized leaf broth mediated nanoparticles and its radical scavenging activity of the present study has efficient than compare to extracts. E.agallocha mediated silver nanoparticles exhibited 82% radical scavenging activity at 40µg of concentration. This is highly comparable to 45% of DPPH radical scavenging activity of 50µl Aedes albopictus mediated silver nanoparticles.21 Preethi & Manusami22 estimated only 50% of DPPH scavenging activity by 12.5mg of Prunus armeniaca mediated silver nanoparticles.
E. agallocha mediated nanoparticles synthesis is fast, cost effective and economically reasonable one. Because in this case we use water, leaf extract and silver nitrate for the nanoparticles synthesis without use of high pressure energy, toxic chemical and the plant metabolites itself acts as a stabilizing agent. The optimized dosage showed significant free radical scavenging activity. Further studies will be needed to find out the exact mechanism of action.
The authors are grateful to the authorities of Annamalai University, Tamil Nadu, India for providing all support during the study period.
The authors declared there is not any conflict of interest.s

©2014 Satyavani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.