Journal of
eISSN: 2373-437X


Short Communication Volume 1 Issue 4
1Vitebsk State Medical University, Belarus
2Department of Microbiology, Vitebsk State Medical University, Belarus
Correspondence: Viktoryia Ziamko, Vitebsk State Medical University, Vitebsk, Belarus-210009, Belarus, Tel 375-29-146-07-99
Received: April 11, 2014 | Published: July 31, 2014
Citation: Ziamko V, Okulich V. Method of isolation of peptidoglycan as a basis for measuring murein–destroying activity of blood serum. J Microbiol Exp. 2014;1(4):144-146. DOI: 10.15406/jmen.2014.01.00025
Determining the activity of enzymes those destroy peptidoglican is the important part of the bacterial cell wall that can substantially help in diagnosis of bacterial infections. We propose the method of separation of a cell wall from gram-positive bacteria, which significantly simplifies and allows reducing cost of the isolation of peptidoglycan, which can be used as substrate for measuring activity of such peptidoglycan-destroying enzymes.
Keywords: gram-positive bacteria, peptidoglycan, enzyme activity, congo red
AMP, antimicrobial peptides; PG, peptidoglycan; PLCR, peptidoglycan labeled with 2% solution of congo red; pkat, picokatals; Еоp, optical density.
Two classes of substances- endogenous antimicrobial peptides (AMP) and endogenous antimicrobial proteins play a very important role in the protection of organism against microbial agents.1,2 Antimicrobial proteins have rather large size containing more than 100 amino acids and most often show lytic enzymatic activity, ability to bind nutrients for bacterial cells or contain sites directed against specific microbial macromolecules. AMP have smaller size and as a rule, destroy the structure or the function of cellular membrane of microorganisms.2–4
Nowadays, there are hundreds of AMP found in epithelial tissues, phagocytes and biological liquids. Some AMP are synthesized constantly, while the synthesis of others is induced in response to an infection or an inflammation. The majority of AMP is presented by cationic granules associated peptides with affinity to components of a microbial cellular wall, for example peptidoglycan.5
When monitoring a course of infectious and inflammatory disease in clinical laboratory practice, elevation of the AMP levels can be useful as marker of system activation of neutrophils.6 The knowledge of the role of enzymes, destroying peptidoglycan during infectious process is very important for understanding of deep mechanisms of interaction between microorganism and immune system of a macroorganism. This is necessary for development of new methods of diagnostics and treatment of infectious diseases.
In our opinion, there is scientific and practical interest in developing new methods of determination of serum activity of the peptidoglycan-destroying enzymes and assessment of their activity in course of various pathological processes.
Purpose: The purpose of this method is to isolate peptidoglycan from the cell wall of gram-positive bacteria and to analyze enzymatic activity of blood serum to destroy the isolated peptidoglycan in patients with purulent otitis.
Isolation of peptidoglycan from gram-positive bacteria
Isolation of PG from cell wall of gram-positive bacteria was carried out by a method proposed by V. Lvov, B. Pinegina and R. Khaitov in our modification.3 Microccocus luteus ATCC 10240 was used as a culture. The isolated PG, labeled with 2% solution of Congo red was used as a substrate for determining the activity of enzymes that destroy PG.7 The quality of the received PG has been determined by means of confocal microscope (Figure 1). We didn’t remove teichoic acids, as it is known that their removal changes the specificity of bacteriolytic enzymes.

Isolation of peptidoglycan is carried out as follows:
Qualitative and quantitative characteristics of the isolated peptidoglycan were evaluated by confocal microscopy. Propidium iodide was added to the reaction mixture in the concentration of 20g/ml. Further, a preparation of “hanging drop” was prepared. Stratified scanning was carried out immediately on confocal microscope Leica TCS SPE.
Scanning was performed in XYZ regimen by using 2 consequential spectral channels. These spectral channels were selected with characteristics such that there was no mutual overlap of the fluorescence from different dyes. The first channel (488nm laser, spectral detection area of 500-530nm) was used for excitation and detection of fluorescence FITC. The second channel (532nm laser, spectral detection area is 570-650nm) was used for propidium iodide. Scanning was performed with a resolution on the plane 150nm, with a distance of 500nm between the layers. The time interval between scans was 3 minutes (Figure 1).
Determining peptidoglycan-destroying activity of blood serum in patients with purulent-inflammatory diseases
18 blood sera were taken from patients with purulent-inflammatory diseases and 18 sera were taken from healthy donors. Patients with purulent-inflammatory diseases were divided in 2 groups: 10 people with acute purulent otitis and 8 with chronic purulent otitis.
The serum was centrifuged 1.5 thousand/ min for 10 minutes. 300µl of PLCR solution and 100µl of serum were added into the first series of eppendorfs. 300µl of PLCR solution and 100µl of serum were pre-heated at 56°C for an hour in order to inactivate the complement. This solution was then added into the second series of eppendorfs. Samples containing Tris-HCl buffer at pH 7.4 in an amount of 300µl and 100µl of serum served as a control. All the samples were incubated in thermostat at t=37°C for 24 hours. When enzymes in blood serum destroyed PG, Congo red became soluble changed its color from colorless to red with a maximum spectrum of absorption at a wavelength of 495nm. After incubation, the samples were removed from the thermostat and were centrifuged for 10 min (10thousand/ min; MICRO 120) for the remaining deposition of PLCR. 150µl of the solution was taken from the supernatant in duplicate and transferred into the wells of 96-well polystyrene plate. The plate was placed in a multichannel spectrophotometer F300, where absorbance was determined in the wells in the wavelength of 492nm.
Interjacent result was expressed in units of optical density and was calculated as the difference between the optical densities of the test samples and their corresponding controls.
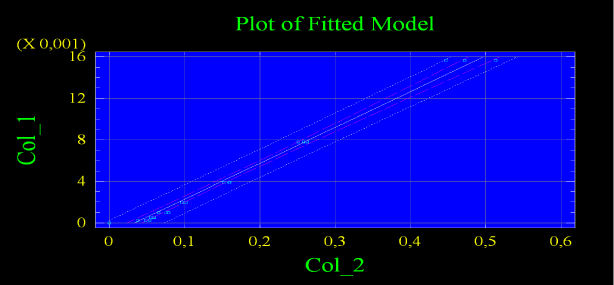
To convert the result into picokatals we used a formula obtained after constructing the calibration graph for the breeding of Congo red, in which the enzyme activity dependence from the optical density of the solution was reflected, assuming that after splitting of one substrate molecule, 1 molecule of Congo red goes into the solution (Figure 2).
Where
Y: the desired result.
Eop: optical density of the sample minus optical density of control.
Since the analysis of the data distribution showed their nonparametric distribution, statistic processing was performed by using the Kolmogorov-Smirnov test in which the differences were considered significant at p<0.05.
Experiment has revealed that peptidoglycan-destroying activity of blood serum in patients with purulent-inflammatory diseases was significantly higher in comparison with donors (p<0.05). After that, the complement inactivation ability of enzymes to destroy PG significantly reduced (p<0.05). Results are presented in the Table 1.
|
S. No |
Group |
N |
Median, pkat |
Percentile, pkat |
Significance of differences |
|
1 |
Donors |
18 |
0.087 |
0.079-0.088 |
P1-2=0.03 |
|
2 |
Patients with purulent otitis |
18 |
0.112 |
0.085-0.136 |
P2-3=0.02 |
|
3 |
Patients with purulent otitis after complement activation |
18 |
0.076 |
0.047-0.099 |
P1-3>0.05 |
Table 1 Peptidoglycan- destroying activity of blood serum before and after complement inactivation in donors and patients with purulent otitis
In our opinion, the results showed that the assessment of activity of peptidoglycan-destroying enzymes can be useful in diagnostics and treatment of infectious diseases.
We would like to thank Pleshkov FI, who contributed in the work and helped to translate it in English.
Authors declare that there is no conflict of interest.

©2014 Ziamko, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.