Journal of
eISSN: 2373-437X


Research Article Volume 12 Issue 3
1Departamento de Ciências Biológicas da Universidade do Estado de Minas Gerais (UEMG), Brasil
2Departamento de Parasitologia e Microbiologia, Universidade Federal do Piauí (UFPI), Brasil
3Departamento de Parasitologia da Universidade Federal de Minas Gerais (UFMG), Brasil
Correspondence: Thelma de Filippis, Universidade do Estadode Minas Gerais, Av. Olegário Maciel, 1427 – Bairro Industrial, Ubá - MG, 36500-000, Brasil, Tel (31)992658182
Received: May 09, 2024 | Published: June 3, 2024
Citation: Filippis T, Barros VC, Melo AL, et al. Characterization of digestive proteases and glycosidases in Lucilia eximia (Diptera: Calliphoridae) larvae: Insights into Dipteran enzymatic processes. J Microbial Exp. 2024;12(2):71-76. DOI: 10.15406/jmen.2024.12.00418
Larvae of Lucilia eximia typically cause secondary myiasis, although they may induce primary myiasis in cats, dogs, and rabbits, possibly transitioning to strict parasitism. To gain a deeper understanding of its physiology larvae were fed with pH indicator dyes mixed with fresh fish to determine the pH of each region of the digestive tract. Glycosidase and protease activities were assessed in homogenates prepared from salivary glands and sections of the digestive tracts of third instar larvae. Excreted/secreted products were extracted from larval-digested fresh fish. Trypsin was the only protease detected in the midgut, absent in salivary glands. Predominant glycosidases identified were α-D-mannosidase and α-D-glucosidase, showing high activity in the midgut and only trace amounts in salivary glands. Lysozyme activity was high in the midgut but low in salivary glands, diverticulum, and excreted/secreted products. This study identifies the major digestive enzymes of L. eximia larvae.
Keywords: Lucilia eximia, digestive enzymes, glycosidases, lysozyme, proteases, excretory-secretory products
As a member of the family Calliphoridae, genus Lucilia includes many important species of flies, such as: L. cuprina that causes primary myiasis in sheep; L. sericata and L. illustris, which are used in larval therapy on infected wounds in patients with osteomyelitis and diabetic ulcers.1 L. eximia (Wiedemann) causes secondary myiasis in domestic animals and human2 and has been found to cause primary myiasis in opossum, cats, rabbits and dogs.3–6 This species is also potentially important in forensics, as it can serve as an indicator for estimating the post-mortem interval of human cadavers,7 especially in outdoor scenes and places with limited accessibility.8,9 L. eximia has often been found in the Neotropical Regions and is commonly found in urban and rural areas of Brazil where it develops in carcasses, garbage, and decaying fruit.10–12 The association of L. eximia with humans in urban and rural areas, as well as forest environments, suggests that this species is a potential vector for transmitting pathogens such as bacteria, viruses, fungi and parasite, similar to Musca domestica.12,13 Although L. eximia is mainly a saprophytic insect and an agent of secondary myiasis, it has the potential to cause primary myiasis, likely due to its preference for the initial stage of decomposition.14 This study, focusing on the digestive enzymes during the development of its larvae, may illuminate the mechanism of the transition to parasitism, considering that the digestive enzymes of saprophytic and parasitic insects are adapted to their specific dietary needs and the nature of the substances they consume.
Serine proteases such as chymotrypsin and trypsin are the major digestive enzymes secreted during skin penetration in some dipteran species that causes primary myiasis (L. cuprina, Chrysomyia bezziana and Dermatobia hominis).26–28
Many digestive glycosidases such as: glucosidases, amylases, mannosidases and lysozymes were mainly detected in insects with different eating habits.29 In L. sericata larvae, α-d-glucosidase, α-D-mannosidase and N-acetyl-β-D-glucosaminidase were described removing sugar residues from the slough/scars proteins, contributing to debridement and sterilizing of chronic wounds.30 Lysozyme is an enzyme that acts on the digestion of bacteria and has been described as a digestive enzyme in Musca domestica larvae.31,32
The aim of this study was to identify some digestive enzymes from L. eximia larvae and their anatomical sites of expression associated with the pH profile of the digestive tube. Investigating the digestive enzymes in the organism of L. eximia larvae is crucial for understanding their physiology and lays the foundation for further studies of this species.
Larvae
Adult L. eximia were collected at the Ecological Station of the Federal University of Minas Gerais, Belo Horizonte, Brazil using a cylindrical trap of fine cloth (15 cm diameter × 40 cm high) supported at both ends by wire hoops. The cylinders were closed at one end, and the trap was hung by the closed end on a tree branch or other appropriate locations. A plastic dish (15 cm diameter) was fixed to the open end of the cylinder with hooks, leaving a space of approximately 1.5 cm between the cylinder and dish. For bait, we used adults Rattus norvegicus carcasses with opened abdomens as attractants, secured inside the dish.33
After 24 h, flies were collected from the traps, and adult L. eximia were transferred to screen cages (30 cm2). In laboratory conditions, the flies were supplied daily with 10% sucrose. Pieces of fish served as substrate for the deposition of eggs and as food for the larvae. Some larvae were allowed to mature to confirm their species. Most studies were conducted using larvae in the early third instar stage because they fed with avidity, and larvae between the first and second phases of development were used to study excreted/secreted products (ESPs).
Analysis of larval gut pH
Vital dye pH indicators, including bromothymol blue (pKa = 7.0), bromocresol purple (pKa = 6.3), and bromocresol green (pKa = 4.6) were combined (4 mg each) with 2 g of macerated raw fish and added to 5 ml plastic bottles containing five larvae. Larvae were dissected 2–4 h after initiating ingestion of the mixture. The gut was removed, and the color of each section of the digestive tract was used to estimate the pH. These colors were compared with those of dyes dissolved in 0.1 M buffer solutions with known pH (Sodium acetate, pH 3.0 to 5.5; MES, pH 5.0 to 6.0 and Tris-HCl, pH 7.0 to 8.0).
Larval excreted/secreted products (ESPs)
To study ESPs, fresh fish served as a substrate, following a methodology similar to that employed by Kaihanfar et al. 2018.34 For the study, 100 mg of substrate was used and incubated in 1.5 ml microcentrifuge tubes, on a water bath at 94 °C for 2 minutes in order to deactivate fish enzymes. Simultaneously, larvae were immersed in Petri dishes with 10 ml of 0.9% saline solution and agitated with the aid of a brush. Subsequently, five larvae were extracted and placed in each microcentrifuge tube containing cooled fish substrate at room temperature (approximately 25 °C) for one hour. After larval removal, 500 µL of 0.9% saline was added to each tube. The supernatant obtained after centrifugation at 10,000g for 2 minutes was assayed for glycosidase and lysozyme activity. Control tubes containing fish meat samples without larvae were used for comparison.
Glycosidase assay
Salivary glands, sections of the midgut (anterior midgut, AM; mid midgut, MM; and posterior midgut, PM) and hindgut were removed and mixed with 50 µL of 0.9% saline and homogenized with the aid of a microhomogenizer. An appropriate volume of 0.9% saline was added to the homogenized material adjusting the total volume to one gland per 40 µL and one gut per 125 µL of the sample. Subsequently, the tubes were centrifuged for 2 minutes at 10,000 xg, and the samples were kept in an ice bath. Glycosidases were assayed using synthetic p-nitrophenyl (PNP) derivatives (Sigma-Aldrich, St Louis, USA) as follows: PNP-D-glucuronide, PNP-N-acetyl-D-galactosamine, PNP-N-acetyl-D-glucosamine, PNP-L-fucopyranoside, PNP-D-galactopyranoside, PNP-D-glucopyranoside, and PNP-D-manopyranoside. An amount of homogenate equivalent to one-half of a gland or one-fifth of the gut was incubated at 30 °C for 1 hour with each substrate (1 mM) in 0.2 M buffer at pH 3.5, 5.0, 6.0 (midgut segments), and pH 5.0, 6.0, and 7.4 (salivary glands), in a volume of 0.5 ml.
The pH of the buffer used in different trials was chosen according to the organ investigated. The buffers (0.2 M and 0.1 M stock and final concentrations, respectively) were as follows: formate–NaOH (pH 3.5), acetate–NaOH (pH 5.0), ethanesulfonic acid (MES)–NaOH (pH 6.0), and sodium phosphate–NaOH (pH 7.4). After incubation period, the reaction was stopped by adding 1 ml of 0.38 M glycine buffer, pH 10.5. The absorbance of the samples at 400 nm was determined using a Shimatzu UV 1650-PC spectrophotometer. Blanks were prepared by adding glycine buffer before adding the substrate. The glycosidase activities of ESPs were determined using these same protocols.
Lysozyme assay
The lysozyme activities of ESP preparations and homogenates of salivary gland, diverticulum, and midgut were determined by measuring the decrease in turbidity of suspensions of Micrococcus lysodeikticus (0.5 mg/ml) following the method described by Lemos and Terra (1991).31 Preparations equivalent to two larvae were incubated for 3 h at 30 ºC with suspensions of M. lysodeikticus prepared in 50 mM sodium phosphate–citrate buffer (pH 3.5, 4.0, 5.0, 6.0, and 7.0) containing 150 mM NaCl in 1 ml. After incubation, the reaction was halted by adding 0.5 ml of 0.5 M sodium carbonate (pH 11.6), and the decrease turbidity was measured at 650 nm before and after incubation using a Shimadzu UV1650-PC spectrophotometer. For assays of the diverticulum it was assumed 50 μl as equivalent to one larva.
Proteolytic activity
Specific inhibitors were employed to determine the types of endoproteases present in larval salivary glands, diverticulum, midgut, and hindgut, using azoalbumin as the substrate. The reaction mixtures (200 µL) comprised 50 µL of 4% azoalbumin prepared in distilled water, 100 µL of buffer (0.1 M acetic acid–NaOH, pH 4.0; 0.1 M MES–NaOH, pH 6.5; or 0.1 M HEPES–NaOH, pH 7.5), 25 µL of the sample (homogenates were equivalent to one-half of a salivary gland, one-fifth of a diverticulum, and one-fifth of a midgut or hindgut), 2 µL of inhibitor, 5 mM mercaptoethanol, and 23 µL water. The mixture was then incubated at 30 ºC for 1 h.
The protease inhibitors included 100 mM TLCK (inhibitor of trypsin-like proteases, Sigma-Aldrich C3142), 100 mM TPCK (inhibitor of chymotrypsin-like proteases, Fluka 93630), 10 mM Pepstatin A (inhibitor of aspartic proteases, Sigma-Aldrich 360325), 10 mM E-64 (cysteine protease inhibitor, Sigma-Aldrich E3132), 100 mM 3,4-dichloroisocoumarin (inhibitor of serine proteases, Sigma D7910) and 5 mM EDTA (inhibitor of metalloproteases, Sigma-Aldrich 431788). The reaction was halted with 600 µL of 10% trichloroacetic acid, and 15 min later the tubes were centrifuged at 10,000g for 5 min. An aliquot of the supernatant (600 µL) was mixed with 700 µL of 1M NaOH, and the absorbance was measured at 440 nm using a Shimadzu UV-1650 PC spectrophotometer. Reactions were also conducted without inhibitors or with all inhibitors present in the same tube.
Anatomical sites of expression correlated with the pH profile of the digestive tube
The midgut can be categorized into three distinct regions based on pH, denoted as anterior midgut (AM), mid- midgut (MM) and posterior midgut (PM) (Figure 1). AM constitutes approximately 25% of the midgut, exhibiting a pH range (from anterior to posterior) of 5.5 to 6.0. The pH within the MM region varies from 3.0 to 4.0. In the PM region, the pH ranges from 4.5 to 6.0 near the pyloric region, subsequently decreasing to a range of 5.0 to pH 5.5. Moving towards the hindgut, there is an increase in pH from 6.0 to 6.5.
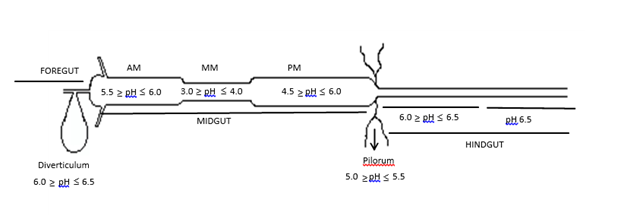
Figure 1 The pH profile of the digestive tract of Lucilia eximia larvae.
AM, anterior midgut; MM, mid midgut; PM, posterior midgut.
Glycosidase activity
Out of the 12 p-nitrophenyl derivatives, only two were significantly hydrolyzed by glycosidases in the larval gut, suggesting the presence of α-D-glucosidase and α-D-mannosidase. These enzymatic activities were identified in AM, MM, and PM, with the highest levels observed in AM and PM (Table 1). The α-D-glucosidase activity, present in all midgut regions, peaked at pH 5.0–6.0, while the α-D-mannosidase activity reached its maximum at lower pH values.
|
|
|
Activity (absorbance values) |
||
|
|
pH |
Midgut segments |
||
|
Enzyme |
AM |
MM |
PM |
|
|
3.5 |
0.285 ± 0.131 |
0.179 ± 0.056 |
0.162 ± 0.058 |
|
|
α-D glucosidase |
5.0 |
0.339 ± 0.035 |
0.227 ± 0.114 |
0.486 ± 0.082 |
|
6.0 |
0.424 ± 0.063 |
0.227 ± 0.048 |
0.635 ± 0.017 |
|
|
3.5 |
1.601 ± 0.435 |
0.438 ± 0.121 |
1.036 ± 0.239 |
|
|
Α-D mannosidase |
5.0 |
1.575 ± 0.225 |
0.390 ± 0.091 |
1.421 ± 0.644 |
|
|
6.0 |
1.195 ± 0.350 |
0.309 ± 0.047 |
0.889 ± 0.239 |
Table 1 Glycolytic activity (absorbance values) in the gut and salivary glands of Lucilia eximia larvae. Activity was standardizes to that of one gut or one salivary gland. (AM, anterior-midgut; MM, mid-midgut; PM, posterior midgut)
The only substrate hydrolyzed by extracts of salivary glands was PNP-α-D-glucopyranoside, indicating the presence of α-D-glucosidase. However, the activity was minimal, displaying only trace levels. ESP preparations showed low levels of α-D-mannosidase activity, but α-D-glucosidase activity was absent. In hindgut homogenates, low activities of α-D-mannosidase and α-D-glucosidase were also detected (Data not shown). As a control, pieces of fish were tested for enzymatic activity, both incubated and not incubated in a hot water bath, revealing that the high temperature inactivated the enzymes from the fish.
Lysozyme activity
Lysozyme activity was found to be higher in the total midgut compared to the salivary gland. Specifically, within the intestine, activity reached its peak between pH 3.5 and 5.0, while in the salivary gland, the optimum range was observed between pH 3.5 and 4.0 (Figure 2). Interestingly, lysozyme activity was noted to be low in ESP at pH 3.5 and 4.0. Moreover, lysozyme activity was detected in AM, MM, and PM, with an optimal pH range between 3.5 and 4.0 (data not shown).
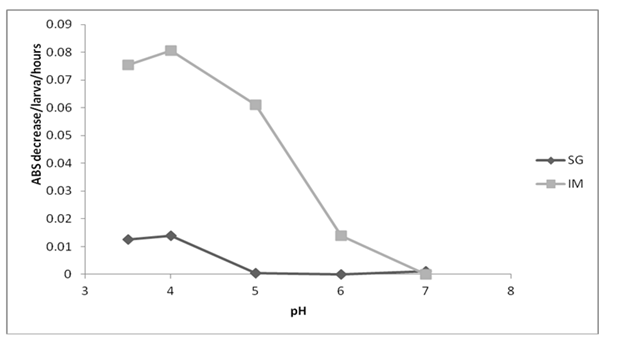
Figure 2 Effect of pH on lysozyme activity of the total midgut (IM) and salivary glands (SG) of third instar larvae of Lucilia eximia. n = two larvae (values standardizes to one larva)
Endoproteases activity
Endoprotease activity assays in the midgut were conducted at pH 4.0, 6.5 and 7.5 (Figure 3). Proteolytic activity was partially inhibited in the presence of the serine protease inhibitors 3,4-dichloroisocoumarin and TLCK, suggesting the presence of a trypsin-like protease. However, it was not inhibited by TPCK, indicating the absence of chymotrypsin or the ineffectiveness of TPCK against larval chymotrypsin (Figure 3). Azoalbumin digestion at pH 4 was not detected, indicating the absence of acid proteases such as cysteine or aspartate proteases. This observation aligns with the findings that pepstatin-A and E-64 (inhibitors of aspartyl and cysteine proteases) did not inhibit overall proteolytic activity. In salivary glands, diverticulum, and hindgut, proteolytic activity was not detected in the presence of EDTA (a metalloprotease inhibitor). While EDTA inhibited protease activity by 23.7% and 11.5% at pH 6.5 and 7.5, respectively, it did not detectably inhibit protease activities in these organs.
The morphology of the digestive tract in L. eximia larvae closely resembles that of other Muscomorpha.35 Tubular salivary glands are linked to the mouth opening. The anterior region of the gut is relatively short and encompasses the pharynx, esophagus, and diverticulum. The diverticulum, a lateral segment of the foregut connected to the anterior part of the esophagus, serves as a storage site for part of the ingested food.
The gut pH fluctuations occur due to the feeding habits of the insect. Larvae of muscomorph dipterans ingest food with large quantities of bacteria, which are killed by the coordinated action of pH and enzymes.15 The utilization of vital dyes incorporated into the food enabled the visualization of pH gradients along the digestive tract. In comparison with other dipterans, both the anterior and posterior midgut in L. eximia larvae exhibit increased acidity, ranging from pH 5.5 to 6.0. This acidity contrasts with the more neutral to alkaline conditions observed in other Muscomorpha diptera, possibly due to the feeding habits of the L. eximia larvae. Further studies are needed to clarify this. The acidic pH in the mid midgut aligns with the feeding habits of larvae that consume bacteria-rich food, creating an environment conducive to the activity of antimicrobial enzymes such as lysozyme and lucifensin.15,25,36
Digestive enzymes play a crucial role in the interaction between parasites and hosts in flies, particularly in cases of myiasis, and serve as targets for the host immune response.37–39 In L. eximia, the secretion of α-D-mannosidase in the midgut, coupled with its absence in salivary glands (except for traces in ESP), suggests that the larvae regurgitate this enzyme during feeding. It is plausible that a portion of the enzyme is transported to the hindgut and excreted in minimal amounts during the final stages of digestion. The observed high activity of α-D-mannosidase aligns with its potential role in digesting bacterial or host glycoproteins.
The second highest enzyme activity detected in the midgut was that of α-D-glucosidase, which presented only traces of activity in the salivary glands. Its pH optimum was 6.0, which was equal to that of the midgut of Cephalopina titillator larvae (Diptera: Oestridae), causing nasal myiasis in camels (camel nasal bot fly).40 In L. sericata, glycosidases were detected in the ESP, removing sugar from slough/eschar proteins, contributing to wound debridement and probably digesting bacteria.30 The activity of α-D-glucosidase was not detected in ESP of L. eximia larvae, indicating that it acts internally, which was consistent with findings that its activity was highest in the posterior midgut. The low level of α -D-glucosidase activity in the hindgut is consistent with the origin in the midgut, because the function of the hindgut is to absorb water and minerals substrate. In adult dipterans such as Anopheles and sandflies that metabolize sugar as an energy source, α-D-glucosidase is the main enzyme detected, and is involved in the digestion of sucrose.41.–43 The presence of α-D-mannosidase in L. eximia ESP, although in low activity, can indicate that the larvae could regurgitate it during the feeding process, likewise in hindgut, probably coming from the midgut and being excreted in low quantities in the final digestion process, along with the α-D-glucosidase. Other study demonstrated that α-glucosidase from Lutzomyia longipalpis, varied its activity according to the substrate. Reports that the microbiota may influence the expression of glycosides on the wound and this could also explain the absence of activity in ESP of L. eximia larvae30. The intestinal microbiota of fly larvae contains thousands of bacteria, such as Enterococcus, Acinetobacter, Providencia, Enterobacter, and Myroides that could be involved in food digestion.44 Further research on the gut microbiota of L. eximia larvae is needed to help clarify the digestive process in this species.
The lysozyme activity detected here could be produced in the midgut, because the highest levels were detected in homogenates of mid midgut. These results indicate that L. eximia larval lysozyme mediates the digestion of decaying matter that contains bacteria. Their acidic pH optima distinguish digestive from nondigestive lysozymes that are normally active at higher pH in the hemolymph.45 This enzyme is present in large quantities in the midgut of M. domestica larvae, and its activity is higher at acidic pH.32,36 The majority of bacteria are destroyed in the midgut of L. sericata.46 The ingestion and digestion of bacteria are important factors that make larvae of L. sericata and L. cuprina useful for the treatment of serious wound infections, and the antimicrobial peptides (defensins) produced by insects that might serve in the future as a novel option for treating bacterial infections in humans.47
The antimicrobial activity of lysozyme from L. eximia needs further investigation to confirm its effectiveness. While chymotrypsin is typically present in larvae of Muscomorpha, such as L. sericata and L. cuprina, our experiment using TPCK as an inhibitor did not detect its presence in L. eximia larvae.23,48 In the midgut of L. eximia larvae, the main protease detected was trypsin-like with perceptible activity measured at pH 6.5 and 7.5. The pH optima for the majority of insect chymotrypsins range between 8–9,17 which may explain the absence of activity of this enzyme in the digestive tube of L. eximia, in which the highest pH was 6.5. The optimum pH is correlated with digestive enzyme activity and ammonia excreted by maggots provide the optimal conditions for serine activity on the wound for debridement.15,49 In Hypoderma lineatum, chymotrypsin was also not detected, being trypsin, the main enzyme found.50,51 The study of cDNA sequences of proteases from the salivary glands of L. sericata did not find a full-length or partial cDNA for chymotrypsin, and the hybridization in situ reveled that it is produced only in anterior midgut.52
Trypsin was not detected in the salivary glands, hindgut, or diverticulum of L. eximia larvae, although it was active in the midgut, particularly in AM and PM, which are tissues with a higher pH. In flies that cause primary myiasis, such as Oestrus ovis, C. bezziana, Hypoderma lineatum, and L. cuprina, trypsin is present in the midgut or ESP, but it is not possible to conclude that chymotrypsin and trypsin are indicators of the specific alimentary characteristics because they are present in flies with variable or specific digestive properties in species such as, C. bezziana, M. domestica , Calliphora vicina and L. cuprina.27,31,53,54
Differences in protease activity could be attributed to differences in affinity for particular synthetic substrates. In L. sericata, the activity of chymotrypsin and trypsin differ depending on the substrate.17 Therefore, purified enzymes of each species must be characterized to determine their specific functions. The activities of other classes of proteases such as aspartyl and metalloproteases were not detected in the digestive tract of L. eximia but are present in ESP of L. sericata and D. hominis.17,28
Maybe the method used (use of inhibitors in a reaction with non-specific substrate) is not very sensitive. It serves well to detect the predominant activities. Possibly other activities could be perceived if we had used specific substrates. L. eximia may be in transition to parasitism and further studies such as those described in this study are important to identify the adaptations that are involved. The present study is the first to identify the activities of the major digestive enzymes of the principal species of the genus Lucilia in Brazil.
Our study provides valuable insights into the digestive enzyme activities of Lucilia eximia larvae, shedding light on their physiological adaptations and potential transition towards parasitism. The characterization of digestive enzymes, including glycosidases, lysozyme, and endoproteases, along with their anatomical localization and pH profiles within the larval digestive tract, underscores the intricate interplay between these enzymes and the larval feeding habits. Notably, the presence of α-D-mannosidase and α-D-glucosidase in the midgut suggests their involvement in digesting bacterial or host glycoproteins, while lysozyme activity highlights its role in combating bacterial colonization. Additionally, the predominance of trypsin-like protease activity suggests its significance in protein digestion, potentially influencing larval feeding strategies. Our findings emphasize the need for further research to elucidate the functional significance of these enzymes and their potential implications in parasitic adaptations of Lucilia eximia.
We are grateful to the funding agencies Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) that supported this study.
The author declares there is no conflict of interest.

©2024 Filippis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.