Journal of
eISSN: 2373-437X


Research Article Volume 4 Issue 6
Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata, Argentina
Correspondence: Maria Celina Elissondo, Laboratorio de Zoonosis Parasitarias, Departamento de Biologia, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata (UNMdP), Funes 3250, 7600 Mar del Plata, Consejo Nacional de Investigaciones Cientificas y Tecnicas (CONICET), Argentina , Tel 54 223 475 2426, Fax 54 223 475 3150
Received: August 02, 2016 | Published: May 15, 2017
Citation: Elissondo MC, Pensel P, Denegri MG (2017) Improvement of the In Vitro Culture of Echinococcus Granulosus Metacestodes. J Microbiol Exp 4(6): 00133 DOI: 10.15406/jmen.2017.04.00133
This work introduces a novel modification to the in vitro experimental vesicular development of the parasite. Our findings showed that protoscoleces exposed to insulin developed into microcysts in shorter times. Moreover, a tenfold increment in the yield of microcysts per tube was observed. An improvement of the in vitro culture of E. granulosus metacestodes was achieved. On the basis of this axenic in vitro system, biochemical, molecular and chemotherapeutical studies of E. granulosus will be greatly facilitated.
Keywords: Echinococcus granulosu, Protoscoleces, Metacestodes, in vitro vesicular development, insulin
A mayor development in parasitology has been the increased interest in attempts to culture parasites outside their hosts. It is well recognized that the value of in vitro techniques is that not only do they allow experiments to be carried out without the use of laboratory animals but also they allow the nutrition, physiology and biochemistry of a parasite to be studied in isolation from the interacting physiology of its host.1
Historically, the primary assessment of antiechinococcal drug candidates has often been performed in rodents, which has led to the extensive use of animal experimentation. Subsequently, the in vitro culture of Echinococcus metacestodes has proven to be a suitable tool for the primary assessment of parasite susceptibility to certain compounds, with a focus on broad-spectrum anti-infective agents, and also represents an ideal model system for studies on drug uptake and associated metabolic changes imposed upon the parasite.2 As the cystic stage of E. multilocularis grows more rapidly than that of E. granulosus, it has proved to be more amenable to culture1 E. multilocularis metacestodes, besides increasing in size, also proliferate asexually and, provided with the corresponding nutrients and growth factors, will form new vesicles either endogenously or exogenously.3
E. granulosusprotoscoleces behaviour in in vitrocultures has been quite well described previously.4-7 A low percentage of protoscoleces develops into microcysts in in vitro systems supplemented with fetal calf serum, independently of their host origin (Table 1). Thus, the amount of metacestodes that could be generated was not sufficient for large-scale drug screening activities.
|
Author |
Geographical origin of samples |
Host of procedence |
Composition of culture medium |
Beginning of vesicularization (In Days) |
Appearance of laminar layer (In Days) |
Appearance of miniature cysts (In Days) |
Obtained cyst (%) |
Maximum survival (In Days) |
|
Present work |
Southeast Buenos Aires Province (Argentina) |
Cattle |
Medium 199 + serum + insulin |
2 |
11 |
14 |
11 |
≥120 |
|
Elissondo et al.10 |
Southeast Buenos Aires Province (Argentina) |
Cattle |
Medium 199 + serum |
5 |
14 |
20-38 |
1.6 |
95 |
|
Elissondo et al.10 |
Tierra del Fuego (Patagonia Argentina) |
Sheep |
Medium 199 + serum |
7 |
14 |
19-27 |
3 |
119 |
|
Rodríguez-Caabeiro6 |
Spain |
Sheep |
Medium 199 + serum |
6 |
9 |
15 |
1 |
90 |
Table 1 Comparison of present and previous works on in vitro vesicular development of protoscoleces of Echinococcus granulosus of sheep and cattle origin
On the other hand, the concept of hormonal cross-communication between evolutionary conserved signaling systems plays an important role in host-parasite interaction in systemic flatworm infections.8 Konrad et al.9 demonstrated that the insulin-receptor-like RTK EmIR can interact with host-derived insulin. Moreover, using the axenic cultivation system, Brehm et al.8 established host cytokines which stimulate RTKs, such as insulin, EGF or FGF, all have positive effects on E. multilocularis growth whereas cytokines of the TGF-β/BMP-family appear to inhibit metacestode growth and rather stimulate protoscolex development.
In order to improve the in vitro culture of E. granulosus metacestodes, the achievement of shorter development times and an increase in the yield of microcysts per tube are needed. The aim of the present work was to study the effect of insulin on the in vitro development of E. granulosus metacestodes.
Protoscoleces of E. granulosus were isolated under aseptic conditions from liver and lung hydatid cysts of infected cattle slaughtered in an abattoir located in the southeast of the Buenos Aires province, Argentina. Viability was assessed by muscular movements (evaluated under light microscope) and by the methylene blue exclusion test.10 The culture protocol was carried out as described previously by Elissondo et al.4 with the addition of different concentrations of insulin to the medium. Briefly, viable and free protoscoleces (1500 per Leightont tube) were cultured in medium 199 (Gibco), containing 60 µg/ml penicillin, 100µg/ml streptomycin, 50µg/ml gentamicin, 4mg/ml glucose and 20% (v/v) fetal calf serum. Insulin (Eli Lilly and Co., USA) was added to the medium resulting in final concentrations of 1.2 and 0.6U ml-1. Cultures without insulin were used as controls. Cultures were performed in 10ml of incubation medium at 37ºC and the medium was changed every 3-4 days. Development was followed microscopically every day. The data recorded for each sample correspond to the moment when a stage was reached by the most advanced individuals of the culture.
Evolution times and yield per tube of control cultures coincides with the previously reported results by our group5 (Table 1). The percentage of obtained cysts was around 1.6%. On the other hand, cultures with the addition of insulin showed some remarkable differences (Table 1). At the beginning of the culture protoscoleces were invaginated (Figure 1a). Vesiculated protoscoleces appeared after 2 days incubation (Figure 1b). After 11 days of culture, a considerable increment in the size of vesiculated protoscoleces could be observed and a laminated layer appeared like a fine membrane in one of the extremes (Figure 1c). On day 14 some microcysts with a complete laminated layer were detected (Figure 1d). By day 20, microcysts completely developed could be observed (Figure 1e). Some cultures could be maintained for more than 120 days when the cultivation was interrupted for an in vitro drug screening experiment (Figures 1f-h). The percentage of obtained cysts per tube was approximately 11% (Table 1). A tenfold increase per tube was registered after the addition of insulin. The used concentrations of insulin on the cultures did not differ in relation to evolution times and yield per tube.
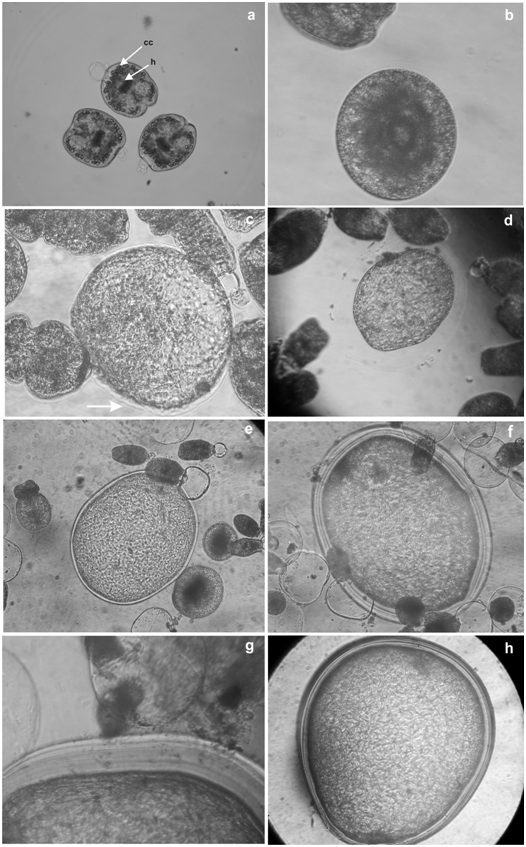
Figure 1 In vitrodevelopment of E. granulosus protoscoleces of cattle origin.
The relationship between parasites and their hosts implies close biochemical co-evolution and communication between their complex physiological and metabolic systems.11 This work describes the effect of insulin on E. granulosus protoscoleces in vitro development to microcysts. On the other hand, this study introduces a novel modification to the in vitro experimental vesicular development of E. granulosus. Our findings showed that protoscoleces exposed to insulin evolved in shorter times. Moreover, a tenfold increment in the yield of microcysts per tube was observed.
Our results are consistent with those reported by Brehm,12 where significant effects of insulin on the formation of E. multilocularis metacestode vesicles from primary cells in vitro were observed. Furthermore, in recent years the effects of insulin on the in vitro development of other cestodes has been reported. Insulin showed significant effects on Taenia crassiceps reproduction, growth, viability and on parasite infectivity.11 In addition, the in vivo response of Mesocestoides vogae to human insulin was demonstrated.13 In the mentioned work, parasite larvae were challenged with different levels of insulin for variable periods. The parameters tested were influenced by human insulin, and suggested a host-parasite molecular dialogue.
We consider that our findings lead to an improvement of the in vitro culture of E. granulosus metacestodes. The achievement of better axenic culture system for E. granulosus metacestodes would introduce several advantages to the study of this parasite. As it was mentioned, theE. multilocularis behavior in vitro cultures is substantially different from E. granulosus. An improvement on the differentiation of E. granulosus metacestode vesicles under host cell free conditions would allow the performance of biochemical and physiological studies without the influence of the intermediate host. For example, different signaling pathways could be investigated. Moreover, the study of the influence of selected host factors on development for prolonged periods of time without the presence of interfering host cells will be possible. Performing of molecular and proteomic studies also would be feasible.
CE is one of the several parasitic diseases of difficult chemical control, where chemotherapy with currently used drugs is highly variable. This disease is included by the WHO in the list of the neglected tropical diseases, in which the use of integrated approaches to cure, prevent and control the disease at the human-animal interface is needed in order to be successful in its prevention and control.14 This in vitro model does not require prior development of secondary hydatid cysts, and thus prevents the use of laboratory animals (which is desirable in itself). The development of cyst using the murine model of cystic echinococcosis last at least 6-8 month after the intraperitoneal inoculation of protoscoleces. This improved in vitro system reduces the time needed for the in vitro drug screening of putative therapeutic agents and could allow the discovery of novel potential drug targets associated with the parasite signalling network.
More exhaustive evaluation of the effect of insulin on E. granulosus should be undertaken. In a next step, we will investigate the effects of the hormone when the parasite is cultured under low oxygen conditions and in the presence of reducing agents.
The authors gratefully acknowledge Dr. Melendez and Sr. Chasma (SENASA, Argentina). This work was supported by the PICT 2012 No. 1164 (ANPCyT, Argentina), PIP 0029 (CONICET) and Universidad Nacional de Mar del Plata (Grant EXA 672/14 y Grant EXA 769/16), Argentina.
None.
There is no conflict of interest.
None.

©2017 Elissondo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.