Journal of
eISSN: 2373-437X


Research Article Volume 4 Issue 6
1Instituto de Ciencias do Mar, Brazil
2Universidade da Integracao Internacional da Lusofonia Afro Brasileira, Brazil
3Universidade Federal do Ceara, Brazil
Correspondence: Vania MM Melo Laboratorio de Ecologia Microbiana e Biotecnologia, Departamento de Biologia, Bloco 909, Centro de Ciencias, Campus do Pici, Universidade Federal do Ceara, Av. Humberto Monte, 2775, 60.440-554 Fortaleza, Ceara, Brazil , Tel 55-85-3366-9814
Received: February 20, 2017 | Published: May 4, 2017
Citation: Tavares TCL, Nogueira VR, Batista GL, Melo VMM (2017) A Sea Hare L-Amino Acid Oxidase to Fight Multiple Antibiotic-Resistant Staphylococcus Aureus. J Microbiol Exp 4(6): 00129. DOI: 10.15406/jmen.2017.04.00129
Staphylococcus aureus is a pathogen notoriously known for its ability to resist antibiotics. Here, we reported the activity of the sea hare Aplysia dactylomela ink L-AAO protein, dactylomelin-P, against 100 clinical isolates of multiple antibiotic-resistance S. aureus with a prevalence of 30% methicillin-resistant (MRSA). Dactylomelin-P was able to inhibit the growth of all isolates with inhibition zones average size of 17.90 ± 2.58 mm. Among the eleven commercial antibiotics tested, only vancomycin, quinupristin/dalfopristin and linezolid exhibited similar efficiency. These findings highlight the potential of dactylomelin-P against S. aureus and MRSA as well as support further research with dactylomelin-P as an antibacterial drug candidate.
Keywords: antibacterial protein, sea hare, MRSA
S. aureusis a serious public health problem associated with superficial wounds to life-threatening infections, with hospital outbreaks and resistant clones emerging frequently.1 Since the 1960s, oxacillin (methicillin)-resistant S. aureus (MRSA) has established itself as one of the most common causes of nosocomial infections, whose dissemination has been of alarming concern.2 Therefore, the interest in discovering novel antibacterial agents with alternative mechanisms of action or unexploited bacterial molecular targets.3 Since the discovery of penicillin, more than 23,000 natural products have been characterized.4 Most of them were small secondary metabolites, however bioactive proteins has emerged concomitantly with advances in new analytical techniques for the isolation and characterization of these biomolecules.
Dactylomelin-P is a 60 kDa monomeric protein purified from the purple ink of the sea hare Aplysia dactylomela that exhibits a wide range antibacterial activity.5 Several sea harescan excrete a deep purple ink when threatened by a predator. Besides pigments of algal origin, the ink is a rich source of toxic peptides and bioactive proteins (Figure 1).
Figure 1 Chemical defense arsenal of the purple ink released by several sea hares.6-15
Dactylomelin-P possess a minimum inhibitory concentration (MIC) of 0.1 mg mL-1 against S. aureus ATCC 25923, with a mechanism of action based on its L-amino acid oxidase (L-AAO) activity against L-lysine and L-arginine.16 This represents a remarkable and economic mechanism of action related with the release of hydrogen peroxide (H2O2) directly on the site of infection as well as other intermediate products generated non-enzymatically with additional bactericide action.17 Considering the activity of dactylomelin-P against S. aureus ATCC 25923 and the increasing MRSA prevalence worldwide, here we examined its activity against 100 clinical isolates of multiple antibiotic-resistant S. aureus with a prevalence of 30% methicillin-resistant (MRSA).
Microorganisms and antibiotics
The microorganisms were kindly provided by the microbiology labs from three public hospitals of Fortaleza City, Ceará State, Brazil: Albert Sabin Hospital; General Hospital of Fortaleza, Walter Cantídio Universitary Hospital, as well as from a private clinical laboratory. These isolates were obtained from abscesses, blood and urine cultures, cerebrospinal and pleural fluid, secretions, catheter tips and mitral valve. Eleven commercial antibiotics were tested against the isolates: oxacillin (1 mg/disk), penicillin (10 UI/disk); clindamycin (2 mg/disk), erythromycin (15 mg/disk), linezolid (30 mg/disk), sulfamethoxazole-trimethoprim (25 mg/disk), vancomycin (30 mg/disk), quinupristin/dalfopristin (15 mg/disk), rifampicin (5 mg/disk), norfloxacin (10 mg/disk) and chloramphenicol (30 mg/disk).
Antibiotic susceptibility test
Dactylomelin-P was prepared at a concentration of 100 mg mL-1 in 0.15 mol L-1 sterile saline. The susceptibility to antibiotics/dactylomelin-P was tested by the disk diffusion method on Mueller-Hinton agar as standardized by the Clinical and Laboratory Standards Institute (CLSI).18
Concerning the source of the isolates, the majority was from blood cultures and secretions (Figure 2). Obtaining 42% of the isolates in blood samples is alarming because S. aureus bacteremia is associated with high case fatality (20-30%).19 All the isolates were penicillin-resistant and sensible to vancomycin, quinupristin/dalfopristin and linezolid (Table 1). The prevalence of MRSA, oxacillin/methicillin resistant, was of 30% and most of the isolates were resistant to multiple antibiotics, which mean they were able to counteract different mechanisms of action (from cell wall synthesis to DNA topoisomerase inhibition). Four of them were resistant to at least seven antibiotics (oxacillin, penicillin, clindamycin, erythromycin, sulfamethoxazole/trimethoprim, rifampicin and norfloxacin). Resistance to erythromycin, sulfamethoxazole-trimethoprim and clindamycin reached 58%, 43% and 28%, respectively. Particularly among the MRSA, the prevalence of resistant to those antibiotics was very elevate (87%, 83% and 60%, respectively) in comparison to the rates reported in previous studies.20-22 Nowadays, it is common to see such resistance profiles, especially against clindamycin, due to haphazard use of these antibiotics.22
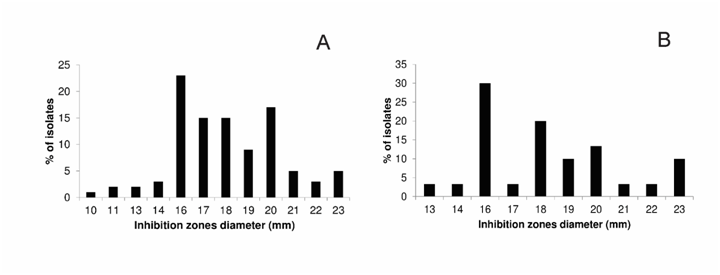
Figure 3 Inhibition zones diameters produced by dactylomelin-P against S. aureus clinical isolates (A) and MRSA strains (B).
aNd-not determined
Source |
Number of strains |
Resistant strains (%) |
|||||||||||
OXA |
PEN |
CLI |
ERY |
LZD |
SXT |
VAN |
QD |
RIF |
NOR |
CHL |
DACT |
||
Wound |
38 |
19 |
100 |
37 |
50 |
0 |
35 |
0 |
0 |
5 |
50 |
50 |
0 |
Catheter |
9 |
55 |
100 |
80 |
33 |
0 |
55 |
0 |
0 |
22 |
50 |
50 |
0 |
Hemoculture |
42 |
38 |
100 |
26 |
65 |
0 |
58 |
0 |
0 |
7 |
30 |
70 |
0 |
Urine culture |
2 |
0 |
100 |
0 |
0 |
0 |
50 |
0 |
0 |
Nda |
Nd |
Nd |
0 |
Abscess |
3 |
0 |
100 |
0 |
67 |
0 |
35 |
0 |
0 |
Nd |
33 |
65 |
0 |
Liquor |
5 |
20 |
100 |
0 |
40 |
0 |
60 |
0 |
0 |
35 |
20 |
0 |
0 |
Mitral valve |
1 |
100 |
100 |
0 |
100 |
0 |
100 |
0 |
0 |
Nd |
0 |
0 |
0 |
Total of strains |
100 |
30 |
100 |
28 |
58 |
0 |
43 |
0 |
0 |
11 |
18 |
11 |
0 |
Table 1 Percentage of S. aureus strains resistant to commercial antibiotics among 100 clinical isolates.
OXA: Oxacillin; PEN: Penicillin; CLI: Clindamycin; ERY: Erythromycin; LZD: Linezolid; SXT: Sulfamethoxazole-trimethoprim; VAN: Vancomycin; QD: Quinupristin/dalfopristin; RIF: Rifampicin; NOR: Norfloxacin; CHL: Chloramphenicol; DACT: Dactylomelin-P
The prevalence of MRSA was 30%, what is alarming in a hospital environment considering that resistance can spread fast. Other studies have also reported high prevalence of MRSA in clinical samples.20-22 Nowadays, vancomycin is used as drug of choice for treating MRSA, but the emergence of vancomycin intermediate-sensitive S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA) has limited even this therapeutic route.2 In these more severe cases, the drugs of choice are quinupristin/dalfopristin, minocycline, daptomycin and linezolid.23-28 Taken together, these issues highlight the importance of searching new drugs to fight such pathogens.
In our study high rates of antibiotic resistance of clinical isolates of S. aureus were observed. Furthermore, our findings highlight the significant antibacterial activity of dactylomelin-P against those isolates including MRSA. Dactylomelin-P, with its alternative L-AAO-based mechanism of action, stood out with a different drug-target interaction, which is very important currently for an effective action against multiple antibiotic-resistant S. aureus. Further studies are necessary to assess the effectiveness of dactylomelin-P for in vivo applications.
There is no conflict of interest.
None.
None.

©2017 Tavares, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.