Journal of
eISSN: 2471-1381


Research Article Volume 4 Issue 4
1Tropical Medicine & Infectious Diseases, Tanta University Faculty of Medicine, Tanta, Egypt.
2Physical Medicine, Rheumatology and Rehabilitation, Tanta University Faculty of Medicine, Tanta, Egypt
Correspondence: AbdElsalam Sherief, Department of Tropical Medicine, Faculty of Medicine, Tanta University, El-Geish Street, Tanta, Egypt, Tel 00201095159522
Received: July 25, 2018 | Published: September 28, 2018
Citation: Magdy M, Badawi R, Abd-Elsalam S, et al. Validity of different nutritional assessment modalities as indicators of nutritional status in cirrhotic patients. J Liver Res Disord Ther. 2018;4(4):145-151 DOI: 10.15406/jlrdt.2018.04.00118
Background & Aim: Nutritional assessment in cirrhotic patients is difficult because many of the traditionally measured parameters, such as weight, body mass index (BMI) and biochemical values vary with the severity of liver disease independently of nutritional status. The aim of this study was determination of the best available methods for assessment of malnutrition in cirrhotic patients and to evaluate the impact of malnutrition on occurrence of complications in those patients.
Methods: One hundred cirrhotic patients were enrolled in this cross sectional study. Nutritional status was assessed by Subjective Global Assessment (SGA), Mini-nutritional assessment (MNA), Controlling nutritional status (CONUT), anthropometry, and dominant hand grip strength (HGS). Statistical analysis was performed using Pearson correlation, Kendall's tau-b and Spearman's rho correlation and Receiver Operating Characteristic(ROC curve) with diagnostic test accuracy measurements(sensitivity, specificity, positive and negative predictive values) using SPSS version 16.0. For all used tests, p<0.05 was considered significant.
Results: One hundred patients (57% male) aged 56 ± 10 years, (Child score C 27%, B 51%, A 22%) were included in our study. Using SGA as a reference method of nutritional assessment, there was high prevalence of malnutrition (88%) in our patients. In an attempt to detect diagnostic accuracy of different nutritional assessment modalities using SGA as reference standard, CONUT was an excellent test for detection of malnourished patients (AUC = 0.91, 95% CI = 0.79-1, P value = 0.0001). MNA was also excellent for detection of malnourished patients (AUC = .0.96, 95% CI = 87-1, P value < 0.0001). Handgrip strength was a very good test for detection of malnourished patients (AUC = 0.83, 95% CI = 0.69-0.97, P value < 0.0001). There was a significant increase in degree of decompensation and occurrence of complications with increasing degree of malnutrition. A cut off value of (18.6, 4.5 and 8.5) for HGS, CONUT and MNA respectively were found to be significant in detecting malnourished patients.
Conclusions: HGS, CONUT, MNA, and dried BMI are compatible to SGA in diagnosis of malnutrition in cirrhosis. Key message: Handgrip strength (HGS) is the best method to assess the nutritional status of the cirrhotic patients as it is non-subjective, not lab dependant, cheap and easy method with high accuracy.
Keywords: Malnutrition, cirrhosis, subjective global assessment, mini-nutritional assessment, controlling nutritional status, hand grip strength
According to the methods of nutritional assessment and degree of severity of liver disease, 65-100% of cirrhotic patients suffer from malnutrition.1 This is may be attributed to cholestasis, presence of porto-systemic shunt, pancreatic insufficiency and bile deficiency with inadequate absorption of long-chain fatty acids and metabolic alterations (high protein catabolism, reduced glucose homeostasis due to alterations of gluconeogenesis, low glycogen stores, pro-inflammatory cytokines such as TNF alpha, interleukins).2
Nutritional status is considered to be a predictor of morbidity and mortality in patients with advanced hepatic disease. Malnutrition in those patients is associated with increased incidence of hepatic encephalopathy, variceal bleeding, refractory ascites, spontaneous bacterial peritonitis (SBP), hepato-renal syndrome (HRS), impaired immunity and increased incidence of infection.3, 4
The ideal tool for nutritional assessment in patients with cirrhosis is challenging due to variation of the traditional tools of assessment that vary with the severity of liver disease independently of nutritional status and the gold standard of nutritional assessment in cirrhotic patients has not been established yet.5
Assessment of nutritional status consists of combination of history, physical examination, laboratory assessment, anthropometrics, and body composition.
Many methods of assessment of nutritional status have been developed to identify malnourished patients or the risk for malnutrition. Most of the methods of assessment like, subjective global assessment (SGA), Mini Nutritional Assessment (MNA), Nutritional Risk screening (NRS) and Malnutrition Universal Screening Tool included blends of information as medical history, dietary intake, amount of weight loss, biochemical variables, and anthropometric measurements.7
The BMI is well known anthropometric measure that is basic in assessment of nutritional state of healthy and diseased persons. It is a cheap and convenient objective tool dependent on height and weight. But, presence of cirrhosis and oedema make it non accurate tool as it overestimate the actual body mass. Campillo8 used the "Dry BMI" as a corrected BMI values interpretation according to amount of ascites.8 They considered these values (Dry BMI) as a valid method of nutritional assessment in cirrhotic patients with sensitivity of 90% and specificity of 86% in their studies Campillo8 Campillo9 Using the interpretation values of dry BMI in our study improved the sensitivity of BMI from 17%-89%, but still with 66% specificity.
Subjective global assessment (SGA) is the most popular method used in evaluation of nutritional state in hospitalized patients.10,11 The use of the SGA was recommended by the 2006 guidelines of the European Society of enteral and parenteral Nutrition (ESPEN) together with anthropometric analysis and handgrip strength test (HGS) for identifying patients with cirrhosis who are at risk of malnutrition.12 Lacking gold standard nutritional assessment tool in cirrhotic patients renders SGA widely accepted tool for evaluation in such patients despite its limitations in predicting clinical outcomes.13,14 So, our study aimed to identify the most efficient tool to assess nutritional state in cirrhotic patients as well as to evaluate the impact of malnutrition on occurrence of complications in those patients.
Our study was designed as a descriptive cross sectional study of 100 patients with cirrhosis, admitted to [removed for blind peer review] starting between June 2016 and February 2017. The diagnosis of cirrhosis was based on the medical history, physical examination, biochemical findings and imaging methods (ultrasound and / or computed tomography).The patients aged 18 years or more with documented liver cirrhosis in a stable hemodynamic condition were invited to enter in our study. Those who accept to share were included. Patients with hepatic encephalopathy grade III-IV, active gastrointestinal bleeding, ongoing alcoholism, sepsis, liver failure, suspected hepatocellular carcinoma (using alpha fetoprotein AFP and / or abdominal ultrasound), chronic diarrhoea, on haemodialysis or with renal failure, patients with chronic debilitating diseases (e.g. DM, T.B) were excluded from the study.
The protocol was approved by the ethical committee of [removed for blind peer review.A written informed consent was obtained from all participants in the study.
The laboratory data collected included complete blood count (CBC), bilirubin, albumin, prothrombin time (PT), International Normalized Ratio (INR), serum urea, serum creatinine and serum cholesterol; all markers were measured by standard laboratory methods. The ultrasound evaluation included the signs of cirrhosis and portal hypertension. For all patients CTP score was calculated based on clinical examination, laboratory findings and U/S data.
Nutritional status of the patients was determined for each patient by means of:
Based on this evaluation, patients were defined as normal, light, moderate and severe degree of under nutrition.
Statistical analysis: The collected data were organized, tabulated and statistically analyzed using Statistical Package for Social Studies (SPSS) version 16. Chicago. SPSS Inc. Categorical data were presented as number and percent while numerical data were presented by mean ± standard deviation for normally distributed data. For skewed data, median and inter-quartile range was used. Association between different parameters and degree of malnutrition was tested using Kendall's tau-b and Spearman's rho correlation to obtain correlation co efficient(r). For evaluation of diagnostic accuracy of different modalities used to test malnutrition in our patients, we perform reservoir operator characteristic (ROC) curve using SGA as the best available reference test. The area under the curve(AUC) with 95%CI were used to get the optimal cutoff values, and sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and test accuracy were calculated accordingly. For all used tests P value <0.05 was considered significant. As SPSS doesn't allow for comparison of ROC curves, we used MedCalc for Windows, version 18.6 (MedCalc Software, Ostend, Belgium) for all pair wise comparison between ROC curves with standard error (SE) calculated according to DeLong et al., 1988. to calculate the Z score. P value <0.05 was considered significant.
A series of 100 hospitalized cirrhotic patients, 57 (57%) male and 43 (43%) female, median age 56 (range 18-76 years) were included. The etiology of liver disease was HCV in 97 (97%), one patient had primary biliary cirrhosis and 2 patients had autoimmune hepatitis. Twenty two patients (22%) were classified as Child A, 51 patients (51%) Child B and the rest 27 patients (27%) were Child C.
By clinical examination of the patients it was found that about 57% of them had ascites in the form of mild, moderate, marked and tense ascites, 64% of them had lower limb oedema, 9% had reducible umbilical hernia, 34% were jaundiced, 54% with pallor, 19% had hepatic encephalopathy grade I & II–in whom nutritional assessment was done after recovery from encephalopathy- and 22% had ecchymosis. The biochemical tests and anthropometric measurements of the studied patients were shown in (Table 1).
The studied patients admitted to the hospital with infection were 52%(16 patients with chest infection,15 with spontaneous bacterial peritonitis, 9 with urinary tract infection, 3 with typhoid and 9 with cellulitis affecting the lower limbs.
Number of malnourished patients according to SGA was 88%, MNA was 88%, CONUT was 95%, handgrip strength was 81%, BMI was 17% and dry BMI was 79% (Figure 1).
In an attempt to detect diagnostic accuracy of different nutritional assessment modalities using SGA as reference standard, dry BMI was a very good test for detection of malnourished patients (AUC = 0.81, 95% CI = 0.66-0.95, P value < 0.0001). CONUT was an excellent test for detection of malnourished patients (AUC = 0.91, 95% CI = 0.79-1, P value = 0.0001).MNA was an excellent test for detection of malnourished patients (AUC = .0.96, 95% CI = 87-1, P value < 0.0001). Handgrip strength was a very good test for detection of malnourished patients (AUC = 0.83, 95% CI = 0.69-0.97, P value < 0.0001) (Table 2) (Figure 2).
We compared the areas under ROC curves using SGA as classification variable to calculate Z score. The result of pair wise comparison of all ROC curves shows that area under ROC curve was significantly higher for MNA in comparison to HGS (P=0.0189) and dry BMI (P=0.0107). All other tests had comparable area under the curve (P>0.05), (Table 3) (Figure 2).
There was a significant positive correlation between degree of malnutrition assessed with Handgrip strength, Dry BMI and presence of infection at time of admission (r= 0.214, 0.212 and P=0.033, 0.035 respectively) while assessment with SGA, CONUT and MNA lacks this correlation (P= 0.225, 0.091, 0.225, 0.237 respectively).There was a significant positive correlation between degree of malnutrition as assessed by scores of CONUT and Handgrip strength and presence of ascites at time of admission (r= 0.190, 0.227 and P= 0.047, 0.024) respectively, while assessment with SGA, MNA and Dry BMI lacks such correlation (P= 0.796, 0.08 and 0.053 respectively)as shown in (Table 4).
Considering severity of liver disease, The degree of malnutrition assessed with SGA, CONUT, MNA, Handgrip strength, Dry BMI had a significant positive correlation to Child score (r= 0.479, 0.292, 0.479, 0.478 and 0.484 respectively), significant negative correlation to serum albumin (r= -0.491,-0.756,- 0.491,-0.558,and -0.443 respectively) with P < 0.0001 for them all, but significant positive correlation to INR for the first 3 methods (r= 0.205, 0.205, 0.279 and P= 0.04, 0.005 and 0.04 respectively)and significant positive correlation to total bilirubin for the first 4 methods (r= 0.376, 0.392, 0.376, 0.288 and P= 0.0001, 0.0001, 0.0001and 0.004 respectively) as shown in Table 4.
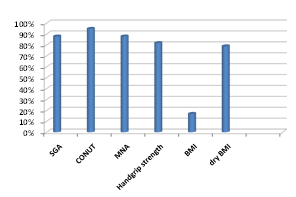
Figure 1 Number of malnourished patients as measured by different modalities of nutritional assessment.
Item |
Mean |
SD |
MIN |
MAX |
Median |
IQR |
Hbgm/dl |
10.33 |
1.97 |
6.1 |
17.2 |
10.1 |
11.1 |
Platelets /cu mm |
134170 |
82006.17 |
20000 |
430000 |
108000 |
410000 |
WBCs /cu mm |
6099 |
2930.88 |
2500 |
13900 |
4950 |
11400 |
TB mg/dl |
2.68 |
2.94 |
0.4 |
13 |
1.65 |
12.6 |
ALT U/L |
39.35 |
35.29 |
10 |
188 |
27 |
178 |
AST U/L |
64.3 |
51.35 |
15 |
274 |
45.5 |
259 |
S. albumin mg/dl |
2.83 |
0.56 |
1.4 |
4.3 |
2.8 |
2.9 |
INR |
1.49 |
0.39 |
1.05 |
3.91 |
1.42 |
2.86 |
S.creatinine mg/dl |
0.98 |
0.3 |
0.21 |
2.04 |
0.995 |
1.83 |
Urea mg/dl |
36.83 |
17.31 |
13.2 |
92 |
32.5 |
78.8 |
S. cholesterol mg/dl |
109.55 |
39.15 |
43 |
256.4 |
104.05 |
213.4 |
ESR1 mm |
37.34 |
22.61 |
5 |
85 |
31 |
80 |
ESR2 mm |
63.99 |
31.48 |
10 |
130 |
60 |
120 |
CRP mg/L |
8.16 |
11.8 |
0 |
48 |
6 |
48 |
RBS mg/dl |
113.08 |
31.44 |
65 |
197 |
103.5 |
132 |
Height |
164.5 |
8.04 |
144 |
189 |
164 |
45 |
Weight |
78.08 |
14.13 |
40.7 |
121.4 |
76.7 |
80.7 |
BMI |
28.95 |
5.47 |
18.1 |
46.3 |
28.25 |
28.2 |
Dry BMI |
1.77 |
0.42 |
18.1 |
46.3 |
2 |
28.2 |
Body fat |
22.13 |
10.45 |
1.1 |
51.5 |
21.65 |
50.4 |
Body water |
39.89 |
7.17 |
13.7 |
55.7 |
39 |
42 |
Table 1 Biochemical tests and anthropometric measurements of the studied patients (n=100)
|
Cutoff |
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
Dry BMI |
18.6 kg/m2 |
89% |
66% |
89% |
57% |
100% |
handgrip strength |
18.6 |
100% |
64% |
65.80% |
100% |
100% |
MNA |
8.5 |
14.20% |
100% |
100% |
98% |
100% |
CONUT |
4.5 |
90% |
100% |
100% |
35% |
91% |
Table 2 Diagnostic accuracy of different nutritional assessment modalities
Compared tests |
Difference |
SE |
95% CI |
Z score |
p |
BMI ~ CONUT |
0.0992 |
0.0589 |
-0.0162–0.215 |
1.684 |
0.0921 |
BMI ~ MNA |
0.148 |
0.0516 |
0.0259–0.243 |
2.637 |
0.0107 |
BMI ~ handgrip |
0.0288 |
0.0213 |
-0.0129–0.0706 |
1.353 |
0.1759 |
CONUT ~ handgrip |
0.0842 |
0.0597 |
-0.0142–0.207 |
1.613 |
0.0798 |
CONUT ~ MNA |
0.0498 |
0.0456 |
-0.0134–0.0912 |
1.534 |
0.0975 |
handgrip ~ MNA |
0.128 |
0.0546 |
0.0211–0.235 |
2.347 |
0.0189 |
Table 3 Pair wise comparison of ROC curves using SGA as classification variable
|
SGA |
MNA |
HGS |
CONUT |
Dry BMI |
|||||
|
r |
P |
r |
P |
R |
P |
r |
P |
r |
P |
Child score |
0.47 |
0.0001 |
0.47 |
0.0001 |
0.47 |
0.0001 |
0.29 |
0.0001 |
0.484 |
0.0001 |
Infection |
0.114 |
0.255 |
0.114 |
0.255 |
0.214 |
0.033 |
0.162 |
0.091 |
0.212 |
0.035 |
Ascites |
0.173 |
0.08 |
0.173 |
0.173 |
0.227 |
0.024 |
0.190 |
0.047 |
0.194 |
0.053 |
Serum albumin |
-0.49 |
0.0001 |
-0.49 |
0.0001 |
-0.55 |
0.0001 |
-0.75 |
0.0001 |
-0.44 |
0.0001 |
INR |
0.205 |
0.04 |
0.205 |
0.04 |
0.188 |
0.061 |
0.279 |
0.005 |
0.121 |
0.229 |
Total bilirubin |
0.376 |
0.0001 |
0.376 |
0.0001 |
0.288 |
0.004 |
0.392 |
0.0001 |
0.192 |
0.055 |
Table 4 Correlation between malnutrition detected by different methods of nutritional assessment and Child score, presence of infection, degree of ascites, serum albumin, INR and total bilirubin
Our In this study, we aimed firstly to identify the most efficient tool to assess nutritional state in cirrhotic patients. SGA is the most popular method used in evaluation of nutritional state in hospitalized patients.10,11 Considering that there is no gold standard test, we used SGA as a reference test.
Using Subjective global assessment (SGA), 88% of our cirrhotic patients were malnourished. This was higher than prevalence reported by Reyes19 (77%) and García-Rodríguez20 (50.9%). This higher prevalence may be attributed to the difference in nutritional habits and lacking regular nutritional evaluation of cirrhotic patients in Egypt.
The severity of chronic liver disease, according to the Child–Pugh classification is correlated to their degree of malnutrition.21 This was true for our patients as a significant positive correlation was found between the degree of malnutrition according to SGA and the child score.
To overcome the long questioner of SGA, reduced forms were developed. Among them Mini-nutritional assessment (MNA) is the commonest. It is the most-established tool internationally to evaluate nutritional status in older people.7 We had used it to assess nutritional status in cirrhotic patients. It was reported that MNA correlated well with nutritional intake and with anthropometric and biological nutritional parameters in tested individuals.22 According to MNA, it was found that the number of malnourished patients was 88%. In our study MNA was able to detect 100% of SGA diagnosed malnourished patients. It was very useful to get the same results obtained with SGA with shorter and easier tool. Our results were in accordance with Yasutake23 who found MNA equal or better than SGA. The severity of liver disease generally correlates with the severity of malnutrition, and protein-calorie malnutrition correlates with worsening of clinical outcome.24 This was in accordance with our study, results which showed as we find a significant positive correlation between the degrees of malnutrition according to MNA the severity of chronic liver disease, according to the Child–Pugh classification.
However, both SGA and MNA had limitations of subjectivity. This is considered a true limitation of the test especially makes the test useless when the patient has some difficulty in reporting their nutritional history adequately.25 This may necessitate the use of more objective tests.
Controlling nutritional status (CONUT) is considered to be an objective tool to assess the degree of malnutrition in cirrhotic patients. It has sensitivity of 90% and specificity of 100% for detection of malnutrition in our patients. This was in accordance with García-Rodríguez20 who considered CONUT a very good test to assess degree of malnutrition in cirrhotic patients with sensitivity 75% and specificity 78.57%.
Cholongitas26 considered the value of CONUT closely associated with severity of chronic liver disease, and one of the most predictive factors for prognosis in cirrhotic patients. In our study there was positive correlation between degree of malnutrition as assessed by CONUT score and severity of chronic liver disease according to Child–Pugh classification. This was in accordance with Taniguchi27 who reported similar results
Controlling nutritional status (CONUT) was an objective test, relatively cheap, had no sophisticated parameters, easily performed and interpreted. All these factors added to its high accuracy render it a very useful tool in assessment of male nutrition in our patients.
However, CONUT may be affected in cirrhotic patients due to the pathology of liver itself which affects the values of albumin and cholesterol (2 of its 3 parameters).This makes lymphocytes the main parameter related to protein depletion and malnutrition indicator).20
Figueiredo28 suggested that screening for body cell mass (BCM) depletion and attenuated muscle function can be measured by Hand grip strength (HGS). In our study, handgrip strength measurement was a very good test for detection of malnourished patients with sensitivity 100% and specificity 64% at cut off value 18.6 kg when compared with SGA. This was in accordance with Daphnee29 who considered HGS a reliable, non-invasive and cost-effective tool to identify malnutrition in cirrhotic patients at cut off value19.5 kg with sensitivity and specificity 67% and 75% respectively. The difference in cut off value of this study and our results may be due to difference in type of handgrip dynamometer used, as Daphnee used Hydraulic Hand Grip Dynamometer while we used portable handgrip Dynamometer.
Our results were in agreement with another study where handgrip strength had a sensitivity and specificity of 86.7% and 70.2%, respectively, for identifying cirrhotic patients with malnutrition.30 HGS has been found to identify 63% of malnourished cirrhotic patients, which is superior to the SGA in the same patients.3 The test is simple and a significant advantage is that the grip strength value is an independent predictor of cirrhosis decompensation.31
In our study, the presence of malnutrition as assessed by HGS was positively correlated to severity of chronic liver disease. Similar results were obtained by Sharma32. It was also associated with increased incidence of infection. This was in accordance with Johnson et al who stated that HGS can predict complications like infection better than the BMI and the SGA.33This may be attributed to the augmented effect of cirrhosis and infection which both produce sarcopenia with subsequent weakness of handgrip strength. This explanation is supported by Reid34 who stated that Infection affects muscle metabolism (inflammatory cytokines and endotoxemia results in increased muscle proteolysis) which resulted in muscle weakness. Handgrip strength is objective, cheap, easy to perform, does not necessitate skilled personnel, and lastly it is considered another quantitative method to assess malnutrition.
So, in conclusion, in our study; using SGA as reference standard: MNA, CONUT, and handgrip strength were the best alternative tests to be used in cirrhotic patients. BMI was not accurate, but using the interpretation values of dry BMI increased its accuracy. There was strong association between the degree of malnutrition and the degree of decompensation.
However, this study has some limitations. The sample size was small, the study was conducted on a selected group of patients with cirrhosis limiting its generalizing ability to all cirrhotic patients especially those with grade III and IV hepatic encephalopathy.
We recommend the use of Handgrip strength (HGS) to evaluate the nutritional status of the cirrhotic patients as it is non-subjective, not lab dependant, cheap and easy method with high accuracy.
None.
The authors declare that they have no competing interests.

©2018 Magdy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.