Journal of
eISSN: 2471-1381


Research Article Volume 4 Issue 4
1Department of Tropical Medicine, Tanta University, Egypt
2Department of Radiology, Ain Shams University, Egypt
Correspondence: Mahmoud Elkadeem, Tropical medicine Department, Tanta University Hospital, El Geish Street, Tanta, Gharbia Governorate, Egypt
Received: August 24, 2018 | Published: November 21, 2018
Citation: Elkadeem M, Salah R, El-Gharib M, et al. Transarterial chemoembolization in patients with hepatocellular carcinoma: study of different outcomes and their predictive factors. J Liver Res Disord Ther. 2018;4(4):157-164. DOI: 10.15406/jlrdt.2018.04.00120
Aim: To analyze the outcomes after transarterial chemoembolization (TACE) including radiological response and liver decompensation, and their predictive factors. Methods: Sixty two hepatocellular carcinoma patients underwent transarterial chemoembolizaton. Laboratory data, tumor criteria, and Child-Pugh score were recorded baseline and at one month post-procedure. Tumor response according to Modified Response Evaluation Criteria in Solid Tumors was evaluated.
Results: Twenty five patients (40.3%) showed complete response, 15 patients (24.2 %) showed partial response, 2 patients (3.2%) showed stable disease and 20 patients (32.3%) showed progressive disease. Significant difference was detected in patients with different radiological responses as regards tumor criteria (size, invasion of portal vein, Barcelona Clinic Liver Cancer stage), and technique of TACE. Thirty six patients (58%) had liver decompensation after TACE. Tumor criteria, serum bilirubin, AST, INR, Model for End Stage Liver Disease, and low platelet count were detected to be predictors of as radiological response and liver decompensation after TACE.
Conclusion: Radiological response cannot be considered alone to determine the outcomes after transarterial chemoembolization. Also, tumor criteria, liver functions, and platelet count are predictors of the outcome.
Keywords: Hepatocellular carcinoma, modified response evaluation criteria in solid tumors, transarterial chemoembolization
Transarterial chemoemolization (TACE) is widely used as a palliative therapy for intermediate stage and unresectable HCC. It has the combination of target tumor chemotherapy and ischemic necrosis due to arterial embolization. TACE is found to improve survival comparing with supportive care.1 Contraindications of TACE are advanced liver disease (Child Pugh≥8), hepatic encephalopathy, refractory ascites, active gastrointestinal bleeding, renal impairment, and advanced tumors (bilobar, main vascular invasion, or extra hepatic metastasis).2 However, patients with extensive disease or deteriorated liver function may have greater risk of complications. The point at which this risk outweighs the benefits remains undefined (Figure 1).3 One of the most important complications of TACE is liver decompensation. Elevation of liver enzymes and negative changes of liver function tests are observed in many patients after procedure. Also evidence of new ascites, hepatic encephalopathy, or worsening of anyone of them which is already present can be observed. The development of the selective and super selective techniques minimized the rates of hepatotoxicity. In patients with preserved liver function, and serum bilirubin < 2.0 mg/dL), the rate of irreversible liver decompensation decreased from 20% in nonselective chemoemblization to 3% in selective or super selective ones.4,5 Radiological response after transarterial chemoemolization (TACE) is classified according to Modified Response Evaluation Criteria in Solid Tumors (mRECIST) to: complete response (CR) (disappearance of arterial enhancement), partial response (PR) ( at least a 30% decrease in the sum of diameters of viable enhancement), progressive disease (PD) (an increase of at least 20% in the sum of the diameters of viable enhancement, or appearance of new lesions), and stable disease (any cases that do not qualify for either partial response or progressive disease).6 However, radiological response is not the only factor predicting TACE outcomes. The best radiological response only does not mean good prognosis.7 Studies detected that other factors (TACE procedure, tumor burden, liver functional status, and health status) had an effect on TACE outcomes.8 Indeed, patients who are eligible for TACE are widely heterogeneous (due to different tumor extension, and different stages of liver functions). Therefore, tailored approach is required to optimize the clinical outcomes in each patient.9 We assessed patients before and after TACE in order to determine which factors might predict TACE outcomes.
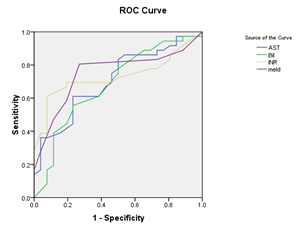
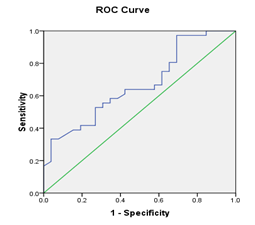
Figure 1 (a) Reciever operating characteristic (ROC) curves show relation baseline serum bilirubin, AST, INR, and MELD score and decompensation after TACE, with area under curve=0.707, 683, 731, 751, and P value =0.006, 0.014, 0.002, 0.001 respectively.(b): Recieveroperating characteristic (ROC) curve shows relation between score and decompensation after TACE. , with area under curve=0.669, P value =0.024 (significant).
This prospective study was carried out on 62 patients in one year period between June 2014 and June 2015. This study was approved by local ethical committee of Tanta Faculty of Medicine; Tanta Faculty of Medicine, Tanta University, Egypt. Hepatocellular carcinoma patients received by Tropical Medicine and Infectious Diseases Department in Tanta University Hospitals, and Interventional Radiology Unit of Ain Shams University Hospitals and were candidate for transarterial chemembolization (TACE) were included in this study. Patients with advanced HCC (extra hepatic metastasis, or main vascular invasion), impaired liver functions (marked ascites, jaundice, or Child C patients), bad general condition, severe bleeding tendency, renal insufficiency, pregnant females, and patients who refused participation in the study were excluded.
Data collection
Informed consent was taken from every patient after explaining the whole procedure. Demographic data were collected. All patients were evaluated before and one month after TACE. Clinical examination, laboratory investigations (including liver, renal functions, complete blood count, alpha-fetoprotein), and radiological investigations including abdomino-pelvic ultrasound and triphasic abdominal CT with contrast were done. Staging of HCC according to BCLC and severity of liver disease (according to Child-Pugh score, and Model for End Stage Liver Disease (MELD) score) were evaluated. Radiological tumor response according to Modified Response Evaluation Criteria in Solid Tumors (mRECIST) assessment was measured for all patients by one radiologist.
TACE procedure
*A 5-french vascular sheath was placed into the common femoral artery over a0.035-inch guide wire. Under fluoroscopic guidance, a5-french glide cobra catheter (Cordis) was advanced into the aorta. An angiographic study of the superior mesentericartery (SMA), celiac trunk and common hepaticartery was performed to identify arterial blood supply of the focal lesion, and evaluate the patency of the portal vein. The arterial branches feeding the tumor were selectively cannulated by microcatheters. Anticancer drug "Doxorubicin hydrochloride” (adriamycin) in oil emulsion was prepared as follows: [dissolving 50 – 100 mg of doxorubicin in 5 ml of a fluid with specific gravity equivalent to that of iodized oil (this fluid was prepared by adding 10 ml of urograffin to 2 ml of distilled water)]. This dissolved doxorubicin was mixed with iodized oil in a ratio of 2-3:1respectively. The doxorubicin in oil emulsion was injected through the catheter into tumor vascular bed under screen. The embolic material gelatin sponge (gel foam) particles 1–3 ml in diameter was injected to occlude the tumor vascular bed and feeding arteries.
Statistical analysis
SPSS (Statistical Package for the Social Sciences) program was used for statistical analysis, (V.16 SPSS Inc, Chicago, IL, USA). Mean and standard deviation were calculated. (T) Tests and one way analysis of variance (ANOVA) were used for normally distributed quantitative data. Mann-Whitney U test, Wilcoxon signed–rank test and Kruskal–Wallis were used for abnormally distributed quantitative data. Pearson Chi-square test and Fisher’s exact test were used for analysis of the qualitative data. Univariate and multivariate analysis were performed using the binary logistic regression model. Significance was established as ρ<0.0. Receiver operating characteristic ROC curve was plotted to predict decompensation after TACE. The validity of the model was measured by the area under the (ROC) curve (AUC).10
Demographic data: The study included 62 patients; 49 (79%) were males and 13 (21%) were females. The overall mean age was 58.24±7.36 years (range: 42-80) (Table 1). Tumor criteria: Most patients 55 (88.7 %) had unilobar tumors, most of them38 (61.3%) were in the right lobe. Thirty one patients (50%) had a single nodule, 19 (30.6%) patients had two nodules, and 12 (19.4%) patients had more than two nodules. The overall mean size of the lesions with 2 dimensions (2D) was 36.23±38.4 cm2 (range: 1.21–180). Twenty patients (32.3%) were Barcelona clinic liver cancer (BCLC stage A), 33 patients (53.2%) were stage B, and 9 patients (14.5%) were stage C (due to tumor invasion of portal vein branch).
Characteristic |
|
Value (mean + SD) (range) / n (%) |
|---|---|---|
Demographic data |
||
Age (mean + SD) (range) |
58.24±7.36 (42-80) |
|
Sex (male / female) |
49 (79) / 13 (21) |
|
HCV |
58 (93.5) |
|
HBV |
1 (1.65) |
|
Both HBV, and HCV |
2 (3.2) |
|
DM |
14 (22.6) |
|
Smoking |
20 (32.3) |
|
Tumor criteria |
||
One |
31 (50) |
|
Number of the lesions |
Two |
19 (30.6) |
Three or more |
12 (19.4) |
|
Rt lobe |
38 (61.3) |
|
Site |
Lt lobe |
17 (27.4) |
Bilobar |
7 (11.3) |
|
One |
34 (54.8) |
|
Segments |
Two |
23 (37.1) |
Three or more |
5 (8.1) |
|
Enhancement (homogenous / non or heterogeneous enhancement) |
37 (59.7) / 25 (40.3) |
|
Size (2D) (mean + SD) (range) |
36.23 ± 38.4 (1.21-180) |
|
Barcelona Clinic Liver Cancer (BCLC) staging |
A |
20 (32.3) |
B |
33 (53.2) |
|
C |
9 (14.5) |
|
TACE procedure |
||
TACE procedure (superselective /selective) |
41 (66.1) / 21 (33.9) |
|
Number of sessions (one / two) |
53 (85.5) / 9 (14.5) |
|
Radiological response |
||
Radiological response (m-RECIST) |
Complete response (CR) |
25 (40.3) |
Partial response (PR) |
15 (24.2) |
|
Stable disease (SD) |
2 (3.2) |
|
|
Progressive disease (PD) |
20 (32.3) |
Table 1 Demographic data, tumor criteria, TACE procedure, radiological response and six-month survival
Transarterial chemoembolization (TACE): Two sessions of TACE were performed in 9(14.5%) patients. However, the remaining 53(85.5%) patients needed only one session. TACE was super selective in 41(66.1%) patients, and selective in 21(33.9%) patients. Radiological response after TACE: According to Modified Response Evaluation Criteria in Solid Tumors (mRECIST), complete response (CR) occurred in 25(40.3%) patients, partial response (PR) was in 15(24.2%) patients, stable disease (SD) was in 2(3.2%) patients, and progressive disease (PD) was in 20 (32.3%) patients.
Laboratory findings: The only significant changes in laboratory findings one month post procedure were increased total serum bilirubin, aspartate transaminase (AST), MELD score, and decreased serum albumin and hemoglobin. There was no significant difference as regards alanine transaminase, INR, alpha-fetoprotein, white blood cells, platelet count, or renal functions (Table 2). As regards classification of patients according to radiological response, no significant statistical differences between groups were detected as regards laboratory findings (except in hemoglobin and platelets), or as regards decompensation one month after TACE. Significant statistical differences were detected in tumor criteria (number of involved segments, vascular invasion, size, BCLC), and selectivity of TACE procedure (Table 3).
Laboratory data |
||||
|---|---|---|---|---|
Baseline (mean ± SD) |
After one month |
Paired t - test |
P value |
|
(mean ± SD) |
||||
Bilirubin (mg/dl) |
1.18±0.43 |
1.49±0.97 |
2.683 |
0.009* |
ALT(IU/L)# |
66.48±48.27 |
56.79 ±35.51 |
1.497 |
0.14 |
AST(IU/L) |
58.66 ±30.03 |
86.7 ± 46.24 |
4.445 |
<0.001* |
Albumin (g/dl) |
3.59±0.46 |
3.16±0.56 |
6.638 |
<0.001* |
INR |
1.2±0.17 |
1.24±0.22 |
1.349 |
0.182 |
MELD score |
9.3 ±2.23 |
10.2±3.02 |
2.783 |
0.007* |
Alpha-fetoprotein |
756.00±2646.5 |
2958.3± 20794.55 |
0.827 |
0.411 |
(ng/ml) # |
||||
Creatinine |
0.85±0.21 |
0.87±0.22 |
0.412 |
0.682 |
(mg/dl) |
||||
Haemoglobin |
12.55±1.93 |
11.79± 1.77 |
4.363 |
0.000* |
WBCs x103 |
5.72 ± 2.33 |
6.15 ±3.39 |
1.366 |
0.177 |
Platelets x103 |
148.1±79.78 |
133.35±73.73 |
1.487 |
0.142 |
Table 2 Comparison of laboratory data, clinical manifestation, and Child Pugh score baseline and one month after TACE
*Significant
# Wilcoxon signed–rank test
Group |
Group I (CR) |
Group II (PR, SD) |
Group III (PD) |
P – value (Fisher,s Exact test) |
|
One |
16 |
6 |
9 |
||
N. of lesions |
Two |
6 |
7 |
6 |
0.406 |
Three or more |
3 |
4 |
5 |
||
Site |
Rt. Lobe |
14 |
12 |
12 |
|
Lt. Lobe |
10 |
3 |
4 |
0.282 |
|
Bilobar |
1 |
2 |
4 |
||
Segment |
One |
19 |
7 |
8 |
0.041* |
Two |
6 |
8 |
9 |
||
Three or more |
0 |
2 |
3 |
||
Enhancement |
Homogenous |
12 |
11 |
14 |
0.289 |
Heterogenous / non enhancement |
13 |
6 |
6 |
(Chi-square test) |
|
Size (2D) (mean ±SD) # |
20.73±14.15 |
40.39±45.0 |
52.07±46.62 |
0.024* |
|
BCLC staging |
A |
10 |
6 |
4 |
0.042* |
B |
15 |
8 |
10 |
||
C |
0 |
3 |
6 |
||
TACE procedure |
Super selective |
23 |
9 |
9 |
0.001* |
Selective |
2 |
8 |
11 |
||
Number of sessions |
One |
24 |
15 |
14 |
0.05 |
Two |
1 |
2 |
6 |
||
Decompensation after TACE |
16 |
9 |
11 |
0.733(Chi-square) |
|
Table 3 Comparison of tumor criteria, TACE procedure, decompensation, and survival between three groups after TACE
*Significant #Kruskal Wallis test
Note BCLC, Barcelona Clinic Liver Cancer; CR, complete respone; PD, progressive disease; PR, partial response; SD, stable disease
In univariate analysis, tumor size, vascular invasion (BCLC stage C), selective TACE, and increase TACE sessions were predictors of progressive disease. Multivariate analysis was run, and revealed that vascular invasion (odds ratio 8.326, ρvalue =0.013 at 95% CI), selective TACE (odds ratio 4.593, ρvalue=0.02 at 95% CI) and increase TACE sessions (odds ratio 6.844, ρvalue =0.024 at 95% CI) were the statistically significant independent factors predictive of PD after TACE. Size (2D) (cm2) with cut off value (40.87), sensitivity 55%, specifity 83.3%, and positive predictive value 61.1% was detected to be associated with progressive disease after TACE (Table 4) (Table 5).
Factor |
Odd ratio |
95% CI |
P- value |
Univariate analysis |
|||
Size of lesion (2D) (cm2) |
1.016 |
1.001-1.031 |
0.039* |
Site (Bilobar, left lobe)/ right lobe |
1.083 |
0.364-3.222 |
0.886 |
Involved segments >1 |
2.437 |
0.82-7.25 |
0.109 |
Number of lesions > 1 |
1.344 |
0.462-3.916 |
0.587 |
Heterogeneous lesions |
1.928 |
0.621-5.985 |
0.256 |
Portal vein branch invasion |
5.571 |
1.225-25.333 |
0.026* |
BCLC (B) / (A) |
1.739 |
0.463-6.533 |
0.412 |
BCLC (C) / (A) |
8 |
1.367-46.812 |
0.021* |
TACE (selective) |
3.911 |
1.262-12.125 |
0.018* |
TACE (2 sessions) |
5.571 |
1.225-25.333 |
0.026* |
Multivariate analysis |
|||
Portal vein branch invasion |
8.326 |
1.551-44.682 |
0.013* |
TACE (selective) |
4.593 |
1.267-16.648 |
0.02* |
TACE (2 sessions) |
6.844 |
1.292-36.258 |
0.024* |
Table 4 Factors associated with progressive disease according to binary logistic regression
*Significant
Decompensation after TACE (increase Child Pugh score one or more than the score before TACE). Decompensation of cirrhosis occurred in 36 (58%) patients. Clinical manifestations and Child Pugh score is shown in Table 5. Univariate analysis was performed to compare patients who remained on the same Child Pugh score after TACE, and those who had Child Pugh score increased for the different pre-procedure variables that might predict decompensation post-procedure. There was no significant difference between them as regards gender, age, and smoking. Comparison of baseline laboratory data was made between them. The mean serum bilirubin, AST, INR, and Model for End Stage Liver Disease (MELD) score were significantly higher, and mean serum platelets was significantly lower in patients who decompensated after TACE. There was a significant difference in the baseline Child-Pugh scores between them. However, there were no statistically significant differences as regards tumor criteria, BCLC staging, and radiological response. From the above univariate analysis, serum bilirubin, AST, INR, MELD score, low platelet count, and also left lobe tumors were the statistically significant factors predictive of decompensation after TACE. Multivariate analysis was run, revealing that AST (ρvalue =0.024 at 95% CI) and INR (ρvalue=0.012 at 95% CI) were the statistically significant independent factors predictive of decompensation after TACE (Table 6).
Clinical manifestations |
||
|---|---|---|
|
Baseline |
After one month |
Ascites |
3 (4.8%) |
22 (35.5%) |
Jaundice |
0 |
22 (35.5%) |
Hepatic encephalopthy |
0 |
5 (8%) |
Child Pugh score |
||
Child Pugh A |
52 (83.8%), |
|
34 (54.8%) with a score of A5, 18 (29%) with a score of A6. |
||
35 (56.5%) |
||
Child Pugh B |
10 (16.2%) (B7) |
23 (37.1%) |
Child Pugh C |
0 |
4 (6.4%) |
Table 5 Comparison of clinical manifestation, and child pugh score at baseline and one month after TACE
Receiver operating characteristic ROC curve was plotted to predict decompensation after TACE. Cut –off values for different laboratory data were calculated (with sensitivity, specifity, and positive predictive value). Serum bilirubin: (cut off value 1.45 mg/dl, sensitivity 38.9%, specifity 88.5 %, and positive predictive value 82%), AST level: (cut off value 77.5 IU/L, sensitivity 36.1%, specifity 96.2%, and positive predictive; value 93%), INR (cut off value 1.2, sensitivity 61.1%, specifity 92.3%, and positive predictive value 92%), Model for End Stage Liver Disease (MELD) (cut off value 8.5, sensitivity 80.6%, specifity 73.1%, and positive predictive value 80.6%), and Low platelet count (cut off value 80x109/L, sensitivity 33.3%, specifity 96.2%, and positive predictive value 92.3%) were detected to be predictors of decompensation after TACE
Factor |
Odd ratio |
95% CI |
P- value |
Univariate analysis |
|||
Bilirubin (mg/dl) |
4.463 |
1.156-17.23 |
0.03* |
ALT (IU/L) |
1.001 |
0.99-1.012 |
0.853 |
AST(IU/L) |
1.031 |
1.008-1.055 |
0.009* |
Albumin (g/dl) (decrease) |
0.877 |
0.292-2.636 |
0.815 |
INR |
935.66 |
9.682-90420 |
0.003* |
MELD score |
1.589 |
1.18-2.14 |
0.002* |
Alpha-fetoprotein (ng/ml) |
1 |
1-Jan |
0.653 |
Platelets (decrease) |
0.992 |
0.985-0.999 |
0.024* |
Child Pugh score > 5 |
0.714 |
0.259-1.971 |
0.516 |
Size of lesion (2D) (cm2) |
1.003 |
0.989-1.016 |
0.706 |
Site (left lobe)/ right lobe |
4.667 |
1.151-18.919 |
0.031* |
Site (Bilobar)/ right lobe |
0.75 |
0.147-3.814 |
0.729 |
Involved segments>1 |
0.933 |
0.339-2.2571 |
0.894 |
Heterogeneous lesions |
3.725 |
1.212-11.449 |
0.022* |
BCLC (B) / (A) |
0.905 |
0.292-2.801 |
0.862 |
BCLC (C) / (A) |
0.833 |
0.17-4.088 |
0.822 |
TACE (Super selective) |
1.059 |
0.365-3.07 |
0.916 |
TACE (2 sessions) |
0.887 |
0.214-3.684 |
0.869 |
m-RECIST (Partial) |
0.633 |
0.18-2.219 |
0.475 |
m-RECIST (Progressive) |
0.688 |
0.207-2.285 |
0.541 |
Multivariate analysis |
|||
AST (IU/L) |
1.03 |
1.04-1.059 |
0.024* |
INR |
581.62 |
4.121-81920 |
0.012* |
Table 6 Factors associated with decompensation after TACE according to binary logistic regression
*Significant
The aim of this study was to analyze the outcomes after transarterial chemoembolizaton (TACE) including radiological response and liver decompensation, and their predictive factors. As regards radiological response, there was a significant statistical difference between mRECIST groups as regard tumor criteria (liver segments involved by tumor, invasion of portal vein branch, size of lesions (two dimensions (2D)), BCLC of the tumor, and technique of TACE. This accords with the study made by Kim;11 this study said that there was significant statistical difference between patients with CR, PR, and PD regarding vascular invasion, number of lesions, and number of TACE sessions. According to the present study, predictive factors of progressive disease (PD) after TACE were size of lesions (2D), portal branch invasion, BCLC (C), TACE technique (selective), and number of TACE sessions. As regards the size (2D): the cut off value was 40.87 cm2 with sensitivity 55%, specifity 83.3%, and positive predictive value 61.1%. Haywood12 stated that there was a significant association between worse mRECIST response and both increasing HCC tumor number, and increasing maximal tumor diameter. Also a study by Chen13 detected that number of nodules and size of tumors was risk factors of tumor progression.14 There are limited studies as regards liver decompensation after TACE because many studies focus on survival and neglect hepatic safety. It is difficult to compare data because of different definitions of liver decompensation in each study, and consideration of TACE as therapeutic option for wide heterogeneous group of hepatocellular carcinoma.15 Decompensation can be defined as occurrence of one of the following: increase ascites, increase in prothrombin time, increase in the serum bilirubin, hepatic encephalopathy and/or deterioration of the Child-Pugh status.16 In this study, TACE has an impact on synthetic liver function. This is shown by significant increase of serum bilirubin, MELD score, and AST, and decrease serum albumin. AST may increase after TACE due to tumor necrosis and ischemic damage.17 Hemoglobin also decreased significantly. However, platelet did not reach the statistical significance. In other studies, platelets showed significant reduction due to increase portal hypertension, and /or drug toxicity.18 Alphafetoprotein failed to decrease, and this could be explained by technical or anatomic issues preventing the treatment from reaching the tumor, aggressive nature of tumor, or undetected tumors present in other parts of the liver or outside it.16
Univariate analysis detected that serum bilirubin, MELD score, low platelet count, and also left lobe tumors, and heterogeneous and non-enhanced lesions were predictive factors of decompensation after TACE. In addition; AST and INR were also detected to be independent predictive factors of decompensation on multivariate analysis. Other tumor criteria and BCLC stages were not predictive factors of decompensation. This is in agreement with Kohla19 who detected significant statistical difference between decompensated patients and those who did not show decompensation as regards serum bilirubin and INR. However; serum albumin, BCLC staging, and number of HCC nodules were also significantly different between groups. Siriwardana20 detected significant difference regarding serum platelets and albumin between those patients who presented with liver failure and those who did not. In this study, decompensation is associated with basal serum bilirubin≥1.45 mg/dl, AST level≥77.5 IU/L, INR≥ 1.2, Model for End Stage Liver Disease (MELD) score≥8.5, and Low platelet count≤ 80x109/L. In a study made by Tasneem;21 decompensation was found to be associated with pre procedure serum albumin levels less than 2.8gm/dl, Child Pugh score>7, and MELD score >10. However, no statistically significant association was observed between liver decompensation and tumor size, transaminases, basal INR, or alphafetoprotein levels.
In the present study, there was no significant statistical difference between decompensated patients and those who did not show decompensation as regards mRECIST, tumor criteria, vascular invasion, and BCLC stage. This is in agreement with another study detected that the complete response did not imply the best outcomes in patients with unresectable HCC, and the radiological response was inadequate as a sole variable to determine the prognosis in patients with HCC who undergo regional therapy.11 Also Trevisani22 reported that tumor response in HCC patients was not found to be a valuable determinant of prognosis. Also Kohla19 detected that BCLC stage, tumor vascular invasion, and extra hepatic spread were not among predictive factors. This might be because decompensation was more dependent on tumor burden and serum albumin, and also presence of small number of patients had vascular invasion in different studies.23–25
The limitations of our study were the small number of patients and that not all patients were evaluated for histological proof of HCC. However, the risk of misdiagnosis was minimized by the fact that our protocol applied the criteria suggested by the EASL for the diagnosis of HCC, for which liver biopsy is not always necessary in the presence of confirming cross-sectional contrast enhanced radiological studies.26 Also, one of the limitations was the lack of data regarding inter-observer variation in the assessment of tumor response to TACE because imaging studies were evaluated by only one radiologist. To summarize, Patients are to be evaluated prior to transarterial chemoembolization as regards liver function, tumor burden, and BCLC stage. Good radiological response cannot be considered alone to as better outcome. Proper patient selection means better outcome, and lower risk of liver decompensation. Tumor criteria (size, left lobe tumors, heterogonous enhanced lesions, and vascular invasion), BCLC stage, TACE technique, serum bilirubin, AST level, INR, MELD score, and low platelet count are independent predictors of TACE outcomes as regards radiological response and liver decompensation.
None.
The author declares that there is no conflict of interest.

©2018 Elkadeem, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.