Journal of
eISSN: 2471-1381


Case Report Volume 3 Issue 7
1Iran University of Medical Sciences, Iran
2Iran University of Medical Sciences, Iran
3Department of Pathology, Iran University of Medical Sciences, Iran
4Department of Radiology, Iran University of Medical Sciences, Iran
5Department of Internal Medicine, Iran University of Medical Sciences, Iran
Correspondence: Marjan Mokhtare, Colorectal Research center, GI fellow and Assistant Professor, Rasoul-e-Akram Hospital, Iran University of Medical Sciences, Tehran, Iran
Received: October 16, 2016 | Published: December 29, 2017
Citation: Ghavam R, Mokhtare M, Mirzaie AZ, et al. Severe hepatotoxic injury and cirrhosis due to acitretin. J Liver Res Disord Ther. 2017;3(7):183-185. DOI: 10.15406/jlrdt.2017.03.00083
We report a case of 48year old man presented with icter, itching and nausea and vomiting. He had developed a severe hepatotoxic reaction, after treatment with acitretin (oral retinoid, which is the derivative of etretinate). Histological findings showed the development of liver fibrosis and cirrhosis. Elevated serum aminotransferase levels, which are usually reversible, have been reported during treatment with acitretin, however; the present study indicates a warning message that severe hepatotoxic injury may follow this treatment.
Keywords: acitretin, hepatotoxicity, cirrhosis, etretinate, hyperlipidemia
LDH, lactate dehydrogenase; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma-glutamyl transferase; MRCP, magnetic resonance cholangiopancreatography
Acitretin, a synthetic retinoid compound, is the active metabolite of etretinate. Due to its more effective pharmacokinetic profile, it is substituted for etretinate as a systemic second-line treatment in severe psoriasis that doesn't respond to topical drugs. Its mechanism of action in psoriasis is to decrease epidermal proliferation. The bioavailability is increased when used with high fat foods.1 Its use is forbidden in women who are pregnant or are planning to be, due to its serious teratogenic effects. Acitretin binds with albumin and is metabolized in the liver. Finally it is excreted through urine and bile.
Patients who receive acitretin, may experience some adverse effects, however, they generally disappear when the dosage is reduced or the drug is stopped. The most common adverse-effects are dry lips and hyperlipidemia that are dose dependent and normally get better during 4-8weeks after the drug discontinuation.2
Pseudo-tumor cerebri is a very rare serious side-effect and acitretin should be discontinued rapidly if the patient experiences relentless, headache, nausea, vomiting and visual disturbance, and neurological assessment performed.3,4 Moreover, vulvovaginitis due to candida has been reported as a rare side effect of this drug.5,6 Increased sensitivity to insulin and hypoglycaemia, are seen in diabetic patients during retinoids utilization.7
Hepatotoxicity as an adverse effect of acitretin is discussed further here. Temporary and generally reversible elevated liver enzymes (LDH, AST, ALT and GGT) may take place in up to 15% of patients who receive acitretin.3,5
Elevated serum alkaline phosphatase (10% to 25%) and direct serum bilirubin (10% to 25%) have been also reported. Acitretin should be disinterested, in the case of toxic hepatic injury during treatment and further examination should be considered.8 Hepatic cell injuries are more prevalent among diabetics, alcoholics and obese persons; therefore evaluation of liver function test is more required in these groups.2 Etretinate could be detected in serum for up to 3years after treatment, probably due to accumulation of the drug in adipose tissue.8
A 48 year old man was admitted in Rasoul-e-Akram hospital with complaint of weakness, pruritus, nausea, vomiting, and also icter; from two weeks ago .He was a known case of pustural psoriasis that confirmed 6 month ago and had received acitretin during the last 4 months. On first physical examination he looked icteric with scratching of the skin due to pruritus and had dry mouth mucosa. He was 10 pack/years smoker. His past medical history was unremarkable. Regarding his family history his father passed away due to liver failure with unknown etiology at the age of 70.The drug was discontinued and the patient received supportive care about acute liver failure.
At admission Liver function test was: AST=1406 U/L (12-38 U/L), ALT=1062 U/L (7-41 U/L), ALP=525 U/L(80-306 U/L), T. Bili=36.6mg/dL (0.3-1.3 mg/dL), D. Bili=17.1mg/dL ( 0.1-0.4mg/dL), Albumin=2.8g/dL(3.5-5.3g/dL), Total Protein =5.9g/dL(6-8.3g/dL),PT=18 s(12.7-15.4 s), INR=1.52 Ratio(1-1.35 Ratio), PTT=38 s(26.3-39.4 s), Gama Glutamyl Teransferase=158 U/L(9-58 U/L), LDH=834U/mL (200-450 U/mL).
Complete blood count (CBC) and renal function test and lipid profile were normal. We found 1+ proteinuria and 3+ bilirubinuria with 2-3 granular casts in urine analysis. The chest X-ray film was normal. The only abnormal finding was coarse normal size liver. At admission, the patient was assessed for viral markers (HBs Ag, HBc Ab, HCV Ab, HIV Ab1, 2, Anti HSV Ab, Anti EBV Ab, Anti VZV Ab, Anti CMV Ab) with negative results and the other laboratory investigations such as; Antinuclear Ab, ASMA, Anti-LKM Ab, serum protein electerophoresis, Ferritin, Anti-mitochondrial Ab, P-ANCA, ASCA, IgG4, TSH, Anti-tissue transglutaminase IgG and IgA Ab) with normal range results. LDH and liver enzyme were decreased during hospital course without any significant change in ALP and Bilirubin level.
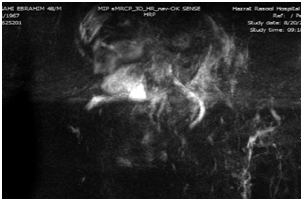
Upper endoscopy was done with normal results. Magnetic Resonance Cholangiopancreatography (MRCP) was done with the result of periportal and pericholecystic edema and decreased intrahepatic bile ducts diameter due to parenchymal hepatic edema. The other parts were normal according to the report (Figure 1).
Finally, liver biopsy was done .The sample was stained with Hematoxylin, and Eosin, Trichrome and Reticulin. Extensive lobular architecture disarray with marked necro-inflammatory changes of parenchyma characterized by obvious hydropic changes and single cell necrosis of hepatocytes as well as infiltration of mixed inflammatory cells was shown in Hematoxylin, and Eosin staining (Figure 2). Collapse of reticulin network associated with psuedolobule formation as well as portal fibrosis was shown in Reticulin and Masson-Trichrome staining (Figures 3&4). Overall findings were in favour of an acute/sub acute liver injury according to Modified HAI score grade: 14/18, stage: 3/6.

The patient with the diagnosis of acitretin induced cirrhosis was referred to liver transplantation clinic. After one week admission course the patient was discharged with relative symptome relief and was requested for monthly follow-up .Five months later in monthly follow-up visit ,these data was found; AST=31 U/L (12-38 U/L), ALT=30 U/L (7-41 U/L) (), ALP=200 U/L (80-306 U/L), Total. Bili=1mg/dL (0.3-1.3mg/dL), Direct. Bili=0.2mg/dL (0.1-0.4 mg/dL), Albumin=3mg/Dl.
Liver fibroscan (Fibroscan model 502 touch, Echosence France ,Paris) was done 5 months later that revealed fibrosis score of F3 (10.1kPa) based on metavir histological index, and steatosis score of 332 (dB/m) which is equal to 80% steatosis, S3 stage. We followed the patient for 9 months without any change in general clinical condition and liver function tests.
The American Academy of Dermatology suggests a protocol for monitoring of these patients, as follows: CBC and renal function tests (baseline and then every 12weeks); lipid profile BS in diabetic patients (baseline and then every 1-2weeks for the first 4-8weeks), and also Liver function test must be evaluated (every 2weeks for the first 8weeks, then every 6-12weeks thereafter;8 if abnormal results would be reported, adjustment of acitretin dose and weekly measurement of liver function test should be considered. In this context, discontinuation of acitretin after three fold increases in transaminases is mandatory, and patients with alanine aminotransferase and bilirubin values more than 200 IU/L and 50mmol/L, respectively should be referred to a gastroenterologist. Monitoring of liver function test for at least 3months is recommended in these patients.9 Acitretin should be disinterested, in the case of toxic hepatic injury during treatment and further examination should be considered.8
Hepatotoxicity as a proved adverse effect of acitretin has been reviewed in some studies. In United State clinical trials, 2/525 patients who received acitretin, had sustained clinical jaundice in association with elevated serum bilirubin and transaminases, but these values returned to normal after discontinuation of the drug. In European clinical trials 2/1289 patients had developed pathology-confirmed toxic hepatitis and based on the second biopsy, fibrotic nodular architecture, suggestive of cirrhosis, was seen in 1 of these patients. In a Canadian clinical trial, 1/63 patients developed hepatotoxicity (3-fold increase in transaminases) and pathology read slight lobular disarrangement, multifocal loss of hepatocytes, and mild inflammation of portal tracts consistent with acute hepatitis. Serum level of liver enzymes in this patient returned to normal values 2months after discontinuation of acitretin. The potential hepatotoxicity after acitretin therapy was prospectively evaluated based on pre-treatment and post treatment liver biopsies. 58%, 25% and 17% of the patients had no change, improvement and aggravation in liver biopsy grades, respectively. No relationship was found between the abnormality of liver function test and the changes in liver biopsies.8 The histopathologic changes after constant use of acitretin are central vein sclerosis, perisinusoidal fibrosis, focal congestion and marked fatty changes.10,11
Due to the rare serious adverse effects of acitretin, it could be prescribed for patients with good compliance and unsuccessful topical treatment for psoriasis. During treatment, regular visits and laboratory monitoring of the patients are necessary. Positive history of liver disease in the first degree family might be considered as a warning sign of susceptibility to hepatic injury among acitretin users.
None.
The authors declare that there is no conflict of interests regarding the publication of this paper.

©2017 Ghavam, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.