Journal of
eISSN: 2471-1381


Case Series Volume 4 Issue 4
1Pediatric Gastroenterologist in Community Hospital of Ures, Mexico
2Military Pathologist in Hermosillo, Mexico
3Endoscopist and General Surgeon, Private practice in Hermosillo, Mexico
4Chemist, Chief, Hospital San Jose, Mexico
Correspondence: Elizondo-Vazquez Jorge Bernardo, Pediatric Gastroenterologist in Community Hospital of Ures, Sonora, México, Health Department of the State of Sonora, Mexico
Received: June 26, 2018 | Published: November 21, 2018
Citation: Bernardo EVJ, Manuel CCJ, Federico PG, et al. Sero-negative celiac disease: a 12-case series. J Liver Res Disord Ther. 2018;4(4):166-169. DOI: 10.15406/jlrdt.2018.04.00121
CD is an immune-mediated systemic disorder elicited by gluten and related prolamines in genetically susceptible individuals and characterized by the presence of a variable combination of gluten-dependent clinical manifestations, CD-specific antibodies, HLA-DQ2 or HLA-DQ8 haplotypes, and enteropathy. CD-specific antibodies comprise auto antibodies against TG2, including endomysial antibodies (EMA), and antibodies against deaminated forms of gliadin peptides (DGP).1 Although the importance of CD auto-antibodies is enormous; the histology is not less important, as a distinct pattern of histological abnormalities has been observed in CD, and it has become clear that a whole spectrum of histological signs may be present, ranging from a normal villous architecture to severe villous atrophy.2 In this series, we describe how CD diagnosis is made through high resolution endoscopy, bulb and descending duodenum biopsies, hematolxylin-eosin staining, immune histochemistry and HLA-DQ2/HLA-DQ8 haplotypes, when CD is suspected by clinical findings, but have negative serologic auto-antibodies.
Keywords: Coeliac disease, immunoglobulin G immunoglobulin A, G anti-gliadin immunoglobulin, A anti-gliadin immunoglobulin, deamidated gliadin peptide G immunoglobulin, deaminated gluten peptide A immunoglobulin, anti-transglutaminase G immunoglobulin, anti-transglutaminase A immunoglobilin, endomysial antibodies, gluten free diet
CD, coeliac disease; IgG, immunoglobulin G; IgA, immunoglobulin A; Anti G-Ig, G anti-gliadin immunoglobulin; Anti A-Ig, A anti-gliadin immunoglobulin; IgG anti-DGP, deamidated gliadin peptide G immunoglobulin; IgA anti-DGP, deaminated gluten peptide A immunoglobulin, IgG anti-TG2, Anti-transglutaminase G immunoglobulin; IgA anti-TG2, anti-transglutaminase A immunoglobilin; EMA, endomysial antibodies, GFD, gluten free diet
This is a series of consecutive clinical cases, with a number of digestive and extra-digestive signs and symptoms, in which the sum of clinical manifestations plus endocopy, histology, haplotypes and remision of signs and symptoms on GFD made the CD diagnosis without positive auto-antibodies for IgA anti-TG2 and IgG and IgA anti-DGP. Once CD is suspected, CD auto-antibiodies are indicated (IgG anti-DGP, IgA anti-DGP, IgA anti-TG2). Blood samples were obtained at CIAD, (Centro de Investigación en Alimentación y Desarrollo [Food and Research Development Center]) in Hermosillo, Sonora, Mexico, which processed the blood samples, and at Hospital San José, in Hermosillo, Sonora, México, which sent the blood samples to a private national reference laboratory in México City. Duodenal biopsies were studied by JMCC; if he suspected CD, he recommended immuno-histopathology to subjects or their parents, if subjects were minors. Follow-up was conducted by JBEV, recopilation of endoscopic images are by FPG and recollection of blood samples by MR.
We included patients subjects of any age, in whom CD was suspected due to clinical findings. Clinical findings are shown in Table 1; CD auto-antibodies are shown in Table 2; and endoscopic, histology and HLA-DQ2/HLA-DQ8 haplotypes findings are shown in Table 3. Signs and symptoms, as shown in Table 1, were predominantly digestive; only one patient reported extra-digestive manifestations exclusively, except for recurrent mouth ulcers; 9 have digestive findings; abdominal distention was the most frequent sign, it might have been the only finding of CD; other patients reported chronic signs and symptoms for years without a diagnosis, in which chronic diarrhea, nausea, vomiting, abdominal distention pain might have been present for years before a diagnosis was made Figure 1, Figure 2. All of the patients are symptomatic, 9 are MARSH 3 A, 2 are 3 B, and 1 is MARSH 2; neither of them are positive for IgA for anti-TG2 and IgA anti-DGP, only four are positive to IgG anti-DGP and eight are negative (Table 1-3).
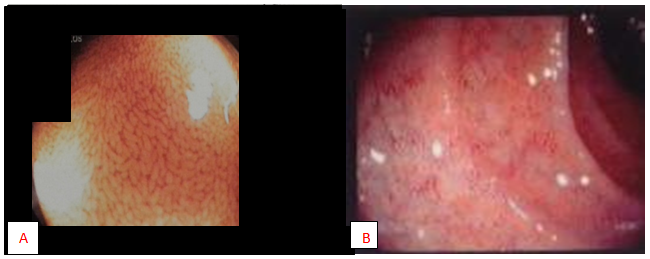
Figure 1 (A) Normal endoscopic villus view of duodenum of a 12 years male with non coeliac, non allergic gluten sensitivity. (B) Endoscopic views of villus atrophy in duodenum in CD.
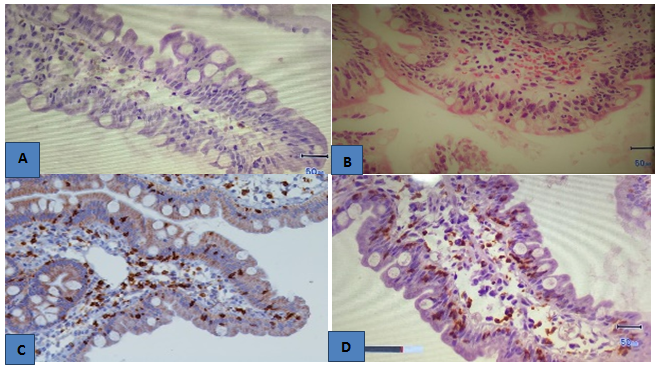
Figure 2 (A) Normal villus of duodenal mucosa with preserved epithelium, goblet cells and lamina propia with scarse lymphocytic infiltration. (B) Hematoxylin eosin stain. Flattened villi of the duodenum, epithelial crypt hiperplasia and lympho-epitheliosis. (C) Immuno-histochemestry for CD3 un duodenal mucosa. Positive lymphocytes are seen on duodenal mucosa and lamina propia. (D) Dense infiltration by CD8+ lymphocytes in duodenal mucosa.
Sex/Age |
Number |
Signs and symptoms |
M/28 y |
1 |
IAC, retro-sternal burn and diarrhea |
F/29 y |
2 |
Herpetic dermatitis, chronic diarrhea and constipation, abdominal pain and IAC |
F/29 y |
3 |
IAC, occasionally watery diarrhea and constipation |
M/60 y |
4 |
IAC, chronic intermittent diarrhea, muscle pain, irritability |
F/30 y |
5 |
Chronic diarrhea, weight loss (10Kg–48 a 38 Kg–in few months) |
F/31 y |
6 |
Herpetiform dermatitis, migraine, allergic rhinitis, arthralagias, recurrent mouth ulcers |
M/9 y |
7 |
Nausea, vomiting, chronic diarrhea, IAC. |
F/24 y |
8 |
Nausea, throw up, chronic diarrhea, abdominal pain, IAC, recurrent mouth ulcers, enamel falling, fibromyalgia, fatigue, herpetiform dermatitis. |
M/52 y |
9 |
Epigastric and retro-sternal burning, chronic diarrhea, IAC, fatigue |
M/6 y |
10 |
Gastric and small bowel dilatation with pseudo-obstruction, failure to thrive and malnutrition, IAC. |
F/65 y |
11 |
IAC, abdominal pain, soar morning taste, chronic nausea and diarrhea |
M/5 y |
12 |
Chronic diarrhea, chronic constipation, IAC. Stomach dilatation |
Table 1 Clinical case, number, signs and symptoms
Note: IAC, increased abdominal circumference
Sex/Age |
Number |
IgG anti-DGP |
IgA anti-DGP |
IgA anti-TG2 |
M/28 y |
1 |
1.4 |
0.7 |
0.6 |
F/28 y |
2 |
0.471/1.8 |
0.914/1.5 |
0.75570.1 |
F/29 y |
3 |
<1.0 |
< 1.0 |
<1.0 |
M/60y |
4 |
>1.0/< 10 |
< 1.0/<10 |
</1.0/< 10 |
F/30 y |
5 |
>1.0 |
<1.0 |
< 1.0 |
F/ 31y |
6 |
<1.0 |
</1.0 |
<1.0 |
M/9 y |
7 |
<1.0 |
<1.0 |
<1.0 |
F/24 y |
8 |
<1.0 |
<1.0 |
<1.0 |
M/52 y |
9 |
<1.0 |
<1.0 |
<1.0 |
M/6 y |
10 |
<1.0 |
<1.0 |
<1.0 |
F/65 y |
11 |
<1.0 |
<1,0 |
<1.0 |
M/5 y |
12 |
1.405 |
<1.0 |
<1.0 |
Table 2 CIAD: Investigation center for food and development, Hermosillo, Sonora, México
Note: Upper normal limit is 1.0. Upper normal limit less than 10: IgA anti-DGP UI/ml, IgG anti-DGP, IgA anti-TG2 UA/mL
Sex/Age |
Number |
Endoscope |
Pathology |
Immuno-histo-chemestry |
HLA-DQ2 |
HLA-DQ8 |
M/28 y |
1 |
NR |
MARSH 3 A |
ND |
ND |
ND |
F/28 y |
2 |
HR |
MARSH 3 A |
ND |
ND |
ND |
F/29 y |
3 |
HR |
MARSH 3 A |
Positive |
Positive |
|
M/60 y |
4 |
NR |
MARSH 3 A |
ND |
Positive |
|
F/30 y |
5 |
NR |
MARSH 3 A |
Positive |
Positive |
|
F/31 y |
6 |
HR |
MARSH 3 A |
ND |
ND |
ND |
M/9 y |
7 |
HR |
MARSH 2 |
ND |
ND |
ND |
F/24 y |
8 |
HR |
MARSH 3 A |
ND |
ND |
ND |
M/52 y |
9 |
HR |
MARSH 3 B |
ND |
Positive |
|
M/6 y |
10 |
NR |
MARSH 3 B |
ND |
Positive |
|
F/65 y |
11 |
HR |
MARSH 3 A |
Positive |
ND |
ND |
M/5 y |
12 |
NR |
MARSH 3 A |
Positive |
ND |
ND |
Table 3 Endoscopic, histology and HLA-DQ2/HLA-DQ8 haplotypes
Note: y, years; NR, normal resolution; HR, high resolution; ND, not done
The understanding of the pathological processes of CD has increased enormously since 1990 to date, leading to a change in the clinical paradigm of CD from a chronic gluten-dependent enteropathy in childhood to a systemic disease with chronic immune features affecting different organ systems; CD may occur at any age.3 A major step forward in the understanding of the pathogenesis of CD was the demonstration in patients with CD of gluten-reactive small-bowel T cells that specifically recognise gliadin peptides in the context of HLA-DQ2 and HLA-DQ8.4 The discovery of TG2 as the major auto antigen in CD led to the recognition of the autoimmune nature of the disease.5 TG2 occurs abundantly in the gut and functions to deamidate proteins and peptides, including gliadin or gliadin fragments, leading to increased T-cell reactivity in patients with CD.6 Tests using DGP as substrate may be of significant value in CD diagnostic testing;7 so IgA and IgG antibodies against TG2, EMA, and DGP are CD-specific auto-antibodies. Evidence is accumulating on the diagnostic value on specific auto-antibodies, associated to the increased frequency of HLA typing for diagnostic purposes; at the same time, the leading role of histology for CD diagnosis has been questioned for several reasons: histological findings are not specific for CD, lesions can be patchy and can occur in the duodenal bulb only, and interpretation depends on preparation of the tissue and is prone to a high inter observer variability.8 CD is characterised by highly specific auto-antibodies directed against the common CD auto antigen TG2,9 and by antibodies against DGP.10 EMA are directed against extracellular TG2.11 Except for DGP antibodies, these antibodies are typically of the IgA class. In IgA-deficient patients with CD, the same type of antibodies in IgG class can be detected.12
Despite the strong arguments in favor of CD-specific auto-antibodies as mentioned above; the association of clinical backgrounds, high-resolution endoscopy, histology and haplo typing constitutes a firm association for CD diagnosis. In this series, all the patients have CD without CD-specific auto-antibodies. Herpetiform dermatitis is not only manifested by blisters, it could be seen as plaques of pruriginous papulae on elbows and thighs, which appear and disappeared over years as occurred in patient 2. Perhaps, the quality determination of CD auto-antibodies may be the difference between the United States and Europe, on the one hand. On the other hand, in Latin America, we still depend, to a great extent, on clinical backgrounds, firstly, and laboratory tests, in second place; thus, we still depend on histological tests for the correct diagnosis of CD.
Finally, this is what is known:
What should be known by clinicians:
None.
The author declares that there is no conflict of interest.

©2018 Bernardo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.