Journal of
eISSN: 2471-1381


Research Article Volume 4 Issue 3
1Department of Biochemistry and Nutrition, Iran
1Department of Clinical Nutrition, Mashhad University of Medical Sciences (MUMS), Iran
2Department of Radiology, Mashhad University of Medicine Sciences (MUMS), Iran
3Gastroenterology and Hematology Research Center, Mashhad University of Medicine Sciences (MUMS), Iran
4Department of Clinical Nutrition, Mashhad University of Medical Sciences (MUMS), Iran
8Department of Basic Medicine Sciences, Neyshabur University of Medical Sciences (NUMS), Iran
9Department of Biochemistry and Nutrition, Mashhad University of Medical Sciences (MUMS), Iran
Correspondence: M Nematy, Department of Biochemistry and Nutrition, Endoscopic and Minimally Invasive Surgery and Cancer Research Center, MD, PhD Associated Professor in Nutrition Science, Faculty of Medicine, Mashhad University of Medical Sciences(MUMS), Mashhad, Iran, Tel 9891-5321-8798, Fax 3628
Received: April 17, 2018 | Published: June 28, 2018
Citation: Razmpour F, Abbasi B, Ganji A, et al. Evaluating the accuracy and sensitivity of anthropometric and laboratory variables in diagnosing the liver steatosis and fibrosis in adolescents with non-alcoholic fatty liver disease. J Liver Res Disord Ther. 2018;4(3):124?128. DOI: 10.15406/jlrdt.2018.04.00114
Background/purpose: Non-alcoholic fatty liver disease is a common chronic disease in this modern world and varied from a silent disease to advanced cirrhosis and hepatocellular carcinoma. Making the diagnosis in the silent stage is valuable in preventing disease progression to progressive phase of the disease. The purpose of this study is evaluation and comparison of sensitivity and specificity of anthropometric parameters and laboratory data in diagnosing fatty liver disease.
Methods: A total sample of 70 overweight and obese children and adolescents were recruited from those admitted to a nutrition clinic in Mashhad, northeastern Iran. The diagnosis of NAFLD was determined by Fibro scan and ultrasound. Anthropometric parameters and liver function test were measured with standard methods. The sensitivity as well as specificity of anthropometric and laboratory values were detected by receiver operating characteristic analysis.
Results: In 70 pediatric and adolescent subjects 37(52.9%) subjects were male and 33(47.1%) subjects were female with an average age of 12.75±2.81 year-old. In detecting liver steatosis, the AUC of BMI (0.764) and WC (0.754) and about liver fibrosis the AUC of WC (0.704) was higher than BMI (0.652). Between laboratory tests AST/ALT had the most AUC (0.604) in predicting liver steatosis. With regarding to liver fibrosis ALT had the most AUC (0.692). In predicting liver fibrosis followed by GGT (0.689), AST/ALT (0.684), AST (0.634) and AST/PLT (0.625). Ultrasound had AUC of 0.69 in predicting liver steatosis and AUC of 0.646 in predicting liver fibrosis. The sensitivity and specificity of ultrasound was only 47.06% and 59.09% respectively for grade one steatosis.
Conclusion: Anthropometric parameters such as waist circumference and BMI have the best performance in diagnosis of liver steatosis and fibrosis in comparison with ultrasonography and laboratory test in adolescents.
Keywords: nonalcoholic fatty liver disease, fibro scan, anthropometric parameter, laboratory variable, adolescence
ALT, alanine amino transferas;
AST, aspartate aminotransferase; GGT, gamma glutamyl trans peptidase; PPV, positive predictive value; NPV, negative predictive value; USS, ultrasonographic steatosis score; CALIPER, canadian laboratory initiative on pediatric reference intervals; TFM, total body fat mass; BMI, body mass index; CAP, controlled attenuation parameter; NAFLD, nonalcoholic fatty liver disease
Non-alcoholic fatty liver disease is a common chronic disease in this modern world that develops as a result of decreased physical activity and consumption of unhealthy foods.1 The prevalence of fatty liver disease has reached from 8-10% to 30% in the last three decades and is reported up to 58% in obese subjects.2‒5 The risk of NAFLD in children as well as adults in greatly increased because of the increasing risk of metabolic syndrome, obesity, insulin resistance and increased blood lipids.6,7
The spectrum of fatty liver can be varied from a silent disease to advanced cirrhosis and hepatocellular carcinoma with overt clinical findings.8 Making the diagnosis in the silent stage is valuable in preventing disease progression to progressive phase of the disease. There are various methods to diagnose fatty liver disease. Biopsy is the gold standard and is often requested in the advanced stages of the disease to differentiate Steatohepatatis from simple steatosis. 9 Ultrasound examination and laboratory studies are commonly used in the early stages and for screening of fatty liver disease.10 The accuracy of ultrasound in the early stages is only 30% and its diagnostic value in the intermediate and advanced stages reaches to 66%11,12 However, the diagnostic accuracy of liver steatosis and determine of the degree of hepatic steatosis in children remain unknown. The accuracy of laboratory tests is reported to be less than ultrasound examination (about 15.4%)10,13 This suggests that we need more accurate measures to diagnosis fatty liver disease in the early stages and before progression to advanced disease. Fibro scan is an imaging method that has traditionally been used to evaluate liver stiffness and hepatic fat deposition.14,15 Controlled attenuation parameter (CAP) has been implemented with the Fibro Scan to assess liver steatosis based on ultrasound attenuation.16 This method is accepted in adult population and proves to be feasible and with acceptable accuracy for detecting steatosis and fibrosis in pediatric population.14 Computed tomography and dual-energy X-ray absorptiometry are not appropriate in pediatric population as they involve ionizing radiation.17
Measurement of body fat and visceral fat has been shown to be useful in diagnosis of NAFLD in different studies.17 Waist circumference and abdominal fat have direct correlation with steatosis and fibrosis in pediatric population and their measurement can be helpful in predicting NAFLD in this population.18 Previous studies have evaluated just one or two diagnostic tools like ultrasound evaluation or biopsy in fatty liver, especially in pediatric population and there is no study to evaluate the value of anthropometric and radiologic studies synchronously. The purpose of this study is evaluation and comparison of sensitivity and specificity of anthropometric parameters and laboratory data in diagnosing fatty liver disease in children.
This is a cross-sectional study on seventy patients of 9-18 year-old with the weight above the 85th percentile for their age that was referred to nutrition clinic. All the subjects were serologically negative for viral hepatitis and autoimmune liver disease. A questionnaire including demographic data like age, gender, history of previous liver disease, history of cardiac disease was filled in. Supine blood pressure was measured from both arms after ten minutes of rest. Anthropometric measurements were performed in suit settings with a flexible ribbon tape with accuracy of 0.01m. Patient's weights were measured with digital scale SECA 704 (made in Germany) with accuracy of 100grams and was recorded in kilograms. The height was measured in standing position with elevated shoulders and in inspiration using a wall tape and was recorded in centimeters. Body mass index (BMI) was calculated by dividing the weight (kg) on the second power of height (m). Waist circumference was measured at the midpoint between the last rib and iliac crest and two centimeters above the umbilicus Leg length was measured from knee to Achilles tendon. All the measurements were recorded in centimeters.
Pediatric and adolescent patients were evaluated by ultrasound examination performed by a pediatric radiologist using an Esoate My Lab class ultrasound device. Liver steatosis in each patient was recorded using a scale of 0 to 3. All subjects underwent fibro scan after a three-hour fasting with an Echosense 504 device and Liver fibrosis and steatosis were evaluated. All magnetic and electronic devices were removed before the study. Steatosis was graded as S0-S3 and fibrosis was grade F0-F4. S0:<237dB/m, S1:237-259dB/m, S2:259-291dB/m, S3: 291-400dB/m مقاله FLI. This method was considered as the standard method for evaluating fatty liver disease. All other measures were compared with this standard.
After 14hours of fasting, 10cc of blood was withdrawn and its serum was separated using Selectra auto analyzer device after 20minutes. All blood samples were collected in a Potassium-EDTA tube and centrifuged for 15minutes in a 3000 rounds per minute speed and liver function tests including Alanine amino Transferas (ALT), Aspartate aminotransferase (AST) , Gamma Glutamyl trans peptidase (GGT) levels were measured.
All data were collected and coded into SPSS v.16. Kolmogorov-Smirnov test was first run to evaluated data distribution. The confidence coefficient was defined as 95% and level of significance of 5% with a power of 80%. We used ROC test to calculate specificity and sensitivity. For each variable, cut off value was calculated. Sensitivity and specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated using MedCalc software.
In 70 pediatric and adolescent subjects 37(52.9%) subjects were male and 33(47.1%) subjects were female with an average age of 12.75±2.81 year-old. The mean of weight was 76.68±15.76kg and the mean of height was 154.41±13cm. The mean of BMI was 28.05±5.074 with no significant difference between male and female subjects. The elastography results for hepatic steatosis was 22(31.42%) healthy, 17(24.28%) grade 1 steatosis, 8(11.42%) grade two steatosis, and 23 (32.85%) grade three steatosis. According to ultrasound examination, 23(32.9%) were healthy, 29(41.4%) had grade one steatosis, 17(24.3%) grade two steatosis, 1(1.4%) grade three steatosis.
According to previous studies, between anthropometric parameters waist circumference and BMI had the most value in predicting the presence of fatty liver and between laboratory data AST, ALT, GGT, AST/Plat and ALT/plat were the most useful tests for primary diagnosis of fatty liver. In this study sensitivity, specificity and ROC under the curve area of these variables were calculated and cut-off points of them were determined. In detecting liver steatosis, waist circumference with the cut-off points of 94.5 had 93.1% sensitivity and BMI with the cut-off points of 28.35 had the sensitivity of 90.62%. The AUC of BMI (0.764) and WC (0.754) was almost similar in predicting liver steatosis (Table1) (Figure 1).
Variable |
Sensitivity |
Specificity |
PPV |
NPV |
ROC Curve |
Cutoff point |
Ultra sound |
80.8 |
56.5 |
79.1 |
59 |
0.69 |
|
BMI |
90.62 |
50 |
60.42 |
86.36 |
0.764 |
28.35 |
Waist Circumference |
93.1 |
48.78 |
56.25 |
90.91 |
0.754 |
94.5 |
ALT |
77.78 |
41.18 |
58.33 |
63.64 |
0.576 |
22.5 |
AST |
61.76 |
25 |
43.75 |
40.91 |
0.426 |
22.5 |
GGT |
64.29 |
25 |
56.25 |
31.82 |
0.342 |
18.5 |
AST/ALT |
57.89 |
79.1 |
22.92 |
63.64 |
0.604 |
1.132 |
AST/PLT |
38.89 |
82.35 |
70 |
56 |
0.348 |
0.0717 |
Table Comparing the sensitivity, specificity, Positive Predictive Value, Negative Predictive Value and ROC curve of diagnosis methods of liver steatosis with gold standard Fibro scan
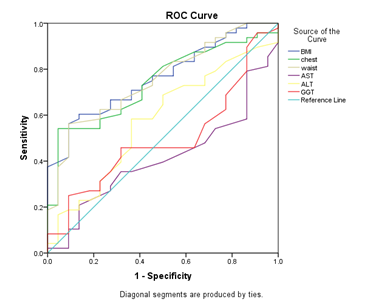
Figure1 Receiver operating characteristic curves (ROC) of anthropometric parameters and serum liver function tests in predicting hepatic steatosis.
In detecting liver fibrosis, waist circumference with the cut-off points of 94.5 had a low sensitivity of 41.4% but a good specificity of 80.49%, also BMI with the cut-off point of 27.3 had a low sensitivity in detecting fibrosis, but the specificity was high enough (78.95%). The AUC of WC (0.704) was higher than BMI (0.652) in predicting liver fibrosis (Table 2) (Figure 2).
Variable |
Sensitivity |
Specificity |
PPV |
NPV |
ROC curve |
Cutoff point |
Ultra sound |
36.1 |
86.9 |
85 |
40 |
0.646 |
|
BMI |
37.7 |
78.95 |
60 |
60 |
0.652 |
28.3 |
Waist Circumference |
41.38 |
80.49 |
60 |
66 |
0.704 |
94.5 |
ALT |
41.67 |
85.29 |
75 |
58 |
0.692 |
23.5 |
AST |
50 |
82.61 |
60 |
76 |
0.634 |
25.5 |
GGT |
66.67 |
52.7 |
75 |
46.2 |
0.689 |
29.5 |
AST/ALT |
66 |
70 |
10 |
66 |
0.684 |
0.869 |
AST/PLT |
80 |
47.3 |
43.75 |
31.82 |
0.625 |
0.068 |
Table 2 Comparing the sensitivity, specificity, Positive Predictive Value, Negative Predictive Value and ROC curve of diagnosis methods of liver fibrosis with gold standard Fibro scan
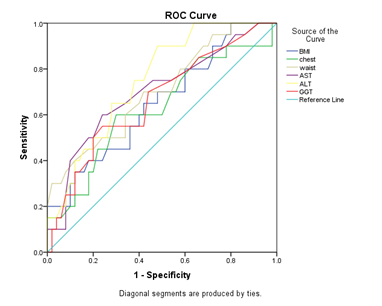
Figure 2 Receiver operating characteristic curves (ROC) of anthropometric parameters and serum liver function tests in predicting hepatic Fibrosis.
Between laboratory tests AST/ALT had the most AUC (0.604) in predicting liver steatosis and with the cut-off points of 1.13 had the sensitivity of 57.9% and specificity of 79.1% After AST/ALT, ALT had the most AUC (0.576) in comparison with other tests and with the cut-off points of 22.5 had the highest sensitivity (77.78%) in predicting liver steatosis (Table 1) (Figure 1). With regarding to liver fibrosis ALT had the most AUC (0.692) in predicting liver fibrosis followed by GGT (0.689), AST/ALT (0.684), AST (0.634) and AST/PLT (0.625). But AST/PLT with the cut-off points of 0.068 had the most sensitivity in detecting liver fibrosis in comparison to other tests followed (Sen=66.67%) (Table 2) (Figure 2).
Between the variables that investigated in this study BMI and then WC had the most AUC in predicting liver steatosis and about liver fibrosis WC and then ALT, GGT and AST/ALT had the most AUC in predicting liver fibrosis.
With regarding elastography as the gold standard, ultrasound had the sensitivity of 80.8%, specificity of 56.5% and AUC of 0.69 in predicting liver steatosis (Table1) (Figure 1).. About liver fibrosis ultrasound had the sensitivity of 36.1%, specificity of 86.9% and AUC of 0.646 in predicting liver fibrosis (Table 2) (Figure 2). The sensitivity and specificity of ultrasound was only 47.06% and 59.09% respectively for grade one steatosis. The sensitivity was even lower for grade two liver steatosis (12.5%).
NAFLD is significantly correlated with metabolic risk factors such as diabetes, inflammation and insulin resistance.19 Some studies showed that NAFLD like metabolic syndrome have a significant relationship with visceral fat disposition.20 In this study we showed that between all of the diagnostic variables, BMI and waist circumference have the most accuracy and AUC in predicting liver steatosis in obese pediatrics and adolescents, with regarding to liver fibrosis in this study waist circumference has the most AUC in predicting liver fibrosis in comparison to other variables, but the accuracy of BMI is lower.
In a study performed by Eskandari et al, the authors suggested that waist circumference can predict visceral fat independent of waist-hip ratio for predict of liver steatosis in adult.21 In another study on pediatric and adolescent subjects, the author mentioned the effect of BMI, waist circumference, hip circumference, and subscapular subcutaneous fat on fatty liver.17,22
Similarly to our results, Subaranian et al showed that the diagnostic power of waist circumference in adults with NAFLD was 0.72.23 Also Vernon G et al.,24 in a study conducted on 2011 reported the power of anthropometric parameters in diagnosing NAFLD in pediatric population and the AUC of waist circumference, total body fat mass (TFM) and visceral fat (IAAF) was 0.720, 0.661 and 0.741 respectively.24 In a study by Rui-Dan Zheng et al. on adult subjects, the BMI was shown to be correlated with visceral fat deposition and liver fibrosis.25
Totally even when the liver functional tests are unnormal, there is the possibility of NAFLD.26 Mehmet had questionable for use of only ALT in diagnosis of steatosis. Shannon et al said that serum ALT and AST are not helpful in obese children in whom fatty liver is suspected.10 Between the transaminase enzymes ALT and AST have clinical value and especially ALT is the indicator of liver injury.27 but based on the international guidelines using the transaminases for diagnosis fatty liver not advised because of their low sensitivity.28 In this study we showed that between laboratory tests AST/ALT and then ALT has the most AUC in predicting liver steatosis. AST/ALT with cut of point (1.13) and ALT with cutoff point (22.5) were in series (Sensitivity=57.9, Specificity=79.1) and (Sensitivity=77.78, Specificity=41.18) respectively.
Burger TS showed in a study on 2006 performed on 72 obese patients with NAFLD that AST level equal or greater than 35 had a 48% sensitivity and 94% specificity for detecting more than 5% steatosis that diagnosed by MRI29 but in this study AST with cutoff point 22.5 was the most sensitivity although was less accuracy than AST/ALT and ALT. In our study we had less cutoff point for transaminases than that study. In a research performed by NHANIS on pediatric population with normal weight and metabolism, 95th percentile of ALT was defined as 25.8U/L for boys and 22.1U/L for girls.30‒32 Similar results were achieved in the Schwimmer’s study, and in a survey on European children.33 Park et al in South Korea defined the 97.5th percentile of ALT as 33U/L for boys and 25U/L for girls.34 Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) defined the normal ALT as 25U/L for 1-12year-old age group.35 The sample value in 24U/L for 13-18 year-old group was defined as 24U/L for boys and 22U/L for girls. The consensus is to consider 25U/L as the upper limit of normal for general pediatric group.31 In our study the highest accuracy and AUC was determined for these levels and the cut-off point of 22.5 revealed to have the highest accuracy, sensitivity and specificity. Also about liver fibrosis ALT has the most diagnostic power which is followed by AST/ALT, AST, AST/PLT respectively.
In a study performed by Hey Ran Yang et al the AUC of AST/PLT ratio for diagnosing hepatic fibrosis was 0.7 and 0.53 for AST/ALT ratio.36 These numbers were respectively 0.625 and 0.684 in our study that was fairly the same as the mentioned study.
Shannon et al.,37 reported the AUC of 0.87 for ultrasound in detecting steatosis.37 A number that was reported as 0.690 in our study with a sensitivity of 80.8% and specificity of 56.5%. We also reported an accuracy of (ROC=0.646), sensitivity of 36.1% and specificity of 86.9%, PPV of 85% and NPV of 40% for ultrasound in detecting liver stiffness. Ultrasonographic steatosis score (USS) had an best correlation with histological grade of fatty liver (with a Spearman’s coefficient of 0.80) but is believed to have diagnostic accuracy in steatosis but not in fibrosis.38 The sensitivity of 80% reported in this method makes it acceptable as a screening tool for NAFLD in early stage. Sadeh et al reported the sensitivity of ultrasound in detecting liver steatosis as 66% for grade two and three, and 30% for grade one.11 We showed a sensitivity of 47.06% and specificity of 59.09% for grade one liver steatosis and 12.5% sensitivity and 100% specificity for grade two steatosis. The result might have been different if we could provide a larger sample volume.
Anthropometric parameters such as waist circumference and BMI have the best performance in diagnosis of liver steatosis and fibrosis in comparison with ultrasonography and laboratory test such as transaminases, While from laboratory tests, AST/ALT and then ALT are better predictor in detecting liver steatosis and with regarding to liver fibrosis ALT and GGT are better predictor in children and adolescents.
Mashhad University of Medical Sciences (MUMS), Mashhad, Iran.
Author declares that there is conflict of interest.

©2018 Razmpour, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.