Journal of
eISSN: 2471-1381


Clinical Paper Volume 3 Issue 7
Department of Surgery, Showa University, Japan
Correspondence: Haytham Gareer, Haytham Gareer, Division of General and Gastroenterological Surgery, Department of Surgery, School of Medicine, Showa University, 1-5-8, Hatanodai, Shinagawa-ku, Tokyo 142-8666, Japan, Tel 142-8666
Received: November 09, 2017 | Published: December 18, 2017
Citation: Gareer H, Murakami M, Aoki T, et al. Laparoscopic intra-glissonian left lateral segmentectomy for hepatic tumors, a gold standard approach. J Liver Res Disord Ther. 2017;3(7):177-181. DOI: 10.15406/jlrdt.2017.03.00082
Background: Laparoscopic left lateral segmentectomy with the intra-glissonian approach has been described as a feasible and safe procedure. We describe the technical procedure and its outcome in terms of feasibility, effectiveness, and safety of a consecutive series of laparoscopic left lateral hepatic segmentectomy. This approach has the advantage of avoiding vascular occlusion throughout the procedure and unnecessary loss of blood and normal parenchyma, while abiding to strict oncologic guidelines and minimizing morbidity and mortality.
Methods: A consecutive series of eligible patients affected by hepatic primary (HCC) and metastatic lesions were selected prospectively to undergo laparoscopic left lateral segmentectomy; Clinical characteristics as well as intra- and postoperative data were prospectively recorded. Secondary outcomes included operating time, intraoperative blood loss/transfusion, and time to resolution of ascites. A follow-up of at least 3months was available for all patients.
Results: Between January 2012 and March 2015, 62 patients (41 men and 21 women) with lesions of the left lateral segment underwent laparoscopic left lateral segmentectomy using this reported resection technique. The mean age of the patients was 64years (range 42-85years) and the indications for resection included primary HCC and colonic metastases. The mean operative time was 172.5min (range 40-365min, P<0.001). All the patients had adequate exposure of the dissection field with blood loss of mean 153ml (range 10-770ml, P<0.001). No limitations, requiring conversions to neither laparotomy, nor intra-operative complications were encountered. The six and 18months overall survival rates were 41.5%(P=1.000) and 34.1%(P=1.000). The six and 18months disease-free survival rates were 72.0% vs. 64.0%(P=1.000).
Conclusions: This technique validates the laparoscopically facilitated left lateral segmental hepatectomy. It utilizes the benefits of the laparoscopic approach while in setting the steps of the conventional open procedure, with the virtue of oncological principles. The procedure is technically feasible, effective, and safe while less time consuming in avoiding the occlusion of the hepatic pedicle at all times.
Keywords: laparoscopic, extra-parenchymal, hepatic lesions, HCC, anatomical resection
Surgical resections are considered the standardized therapy for primary cancers of the liver and for the majority of the metastatic disease affecting this organ.1,2 Outcome of hepatic resections is influenced primarily by massive bleeding during resection and the subsequent need for blood transfusion.3,4 The standard technique to control intraoperative bleeding is The Pringle maneuver;5 however it has the disadvantage of an ischemic-reperfusion injury. This could be particularly prominent in cirrhotic livers, which have little tolerance of anoxia and serum deprivation. Additionally, with this technique there are also associated risks of bacterial translocation, intrahepatic metastases, impaired liver regeneration, systemic inflammation, and multiple organ dysfunction syndromes.6 This technique has been modified to decrease these adverse effects, with measures including intermittent (15min or 30min) occlusion,7 hemihepatic artery control (half-Pringle maneuver),8,9 selective hepatic vascular exclusion for tumors involving the hepatic veins and ischemic preconditioning.10
Resections of a single hepatic segment or of two or more anatomical segments can be safely performed after identifying their afferent vascular pedicles.11 In open segmental resection of the left lateral lobe the segmental branches of segments 2 and 3 are divided extra-hepatically, thus avoiding the need for a Pringle maneuver entirely. This conventional approach facilitates control and ligation of these branches and ensures the prevention of tumor cells dissemination long the portal vein. It also aims to limit morbidity and mortality while implementing a sound oncologic approach in treatment of malignant hepatic lesions.12
The laparoscopic approach for liver resection allows for an even more facilitated incorporation of this technique with reduced morbidity and minimal mortality.13,14 With this report, we aim to validate the laparoscopic left lateral hepatectomy technique, which follows the same principle of its open counterpart and avoids total vascular occlusion throughout the procedure. It makes use of the advantages in performing segmental hepatectomy by a minimal invasive laparoscopic approach.15,16 It also embraces the advantages of the conventional open resection in ensuring limits in cancer cell tendency to disseminate along the portal vein and its intrasegmental branches.17 Thus, it is more oncologically safe, particularly for hepatocellular carcinoma resections.
The technique
Patients were selected for resection on the basis of Makuuchi's criteria.18 This criterion bases the limits of resection on three variables; the absence or presence of ascites; the serum total bilirubin concentration, and the indocyanine green (ICG) clearance rate at 15min. We considered patients for extent of resection based on; the size and location of the lesion (accessed by computed tomography (CT) scans), absence of ascites and a normal bilirubin level (<1mg/dL). The ICG clearance rate was the main determinant of residual liver function. All the patients were evaluated to laboratory investigations which included blood examinations (aspartate transaminase, alanine transaminase, serum albumin, total and direct bilirubin, prothrombin time, and a-fetoprotein). The authors routinely use technetium-99m diethylenetriamine-penta-acetic acid-galactosyl human serum albumin (99mTc-GSA) in predicting residual liver capacity prior to the operation. We also perform spirometry to check pulmonary function and, in cirrhotic patients, an upper GI endoscopy to evaluate esophageal varices and colonoscopy to exclude a colonic primary.
Patient and trocar positioning
The patient is placed supine with both hands by the sides, the operator standing to the right of the patient; the assistant on the opposite side; and the scopist to the left of the assistant. With an open laparoscopy technique, continuous CO2 pneumoperitoneum is induced at a pressure of 12mmHg. Usually we use a five-trocer configuration. A 12-mm port at the umbilicus houses the 30°C laparoscope and another 12-mm trocar just below the subcostal angle for introduction of the Habib Laparoscopic Sealer 4X device (Angio Dynamics, Queensbury, NY, US). The other three trocars are operator trocars and they each host a 5-mm port.
Operative procedure
A standard diagnostic and staging laparoscopy is performed and the liver is examined. Inspection of the peritoneal cavity for ascites and signs of portal hypertension is always conducted. The liver volume, lesion characteristics, and presence of steatosis, cholestasis or cirrhosis can be evaluated. If necessary, laparoscopic ultrasonography is used to confirm the extension of the lesion(s) and relationships to the vasculature.
Mobilization
The falciform, the round, and left triangular ligaments, and the lesser omentum are divided, using a Harmonic scalpel (Ultracision; Ethicon Endosurgery Cincinnati, OH, USA) or monopolar (daithermy hook) coagulation, all the way down until visualization of the suprahepatic inferior vena cava.
Identification of portal branches and venous dissection
Using clearly visible landmarks on the anterior surface of the liver, namely the fissure of the umbilical ligament, we identify the termination of the left portal branch situated at the end of this fissure. From this umbilical/posterior (U/P) point, the right horns of segment 4 and left horn for segment 3 and segment 2 originate. In following this step, it is almost always easy to identify the individual branches, which are enclosed by a sheath of connective tissue. These portal branches run together with their corresponding arterial and biliary branches, enclosed in connective tissue and visible on the left after detachment of Glisson's capsule.
The visceral surface of the liver is exposed by lifting the left lobe of the liver upwards with the assistant using a liver retractor. We start at the umbilical fissure, by gentle tactile transection on the leaflets of the capsule using a curved grasper, to dissecting the tissue overlying to separating segments 3 and 4. Form this point, the clear bifurcation of the portal branches P2 and P3 are individually dissected, clipped and ligated as seen in Figure 1.
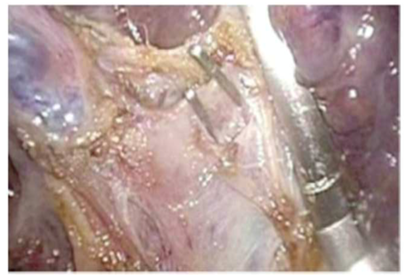
The corresponding left lateral sectional branches of the hepatic artery and the bile duct can then be easily identified in running course form the U/P point. They are controlled and divided within the liver substance after their through identification, thus avoiding the need for a hilar dissection; this gives the advantage of early bleeding control and a virtually bloodless procedure. Thus, identification of the segmental branches of the portal vein and corresponding vessels could be facilitated without the transection of the parenchyma. When identified, these branches are clipped and transected with the Harmonic scalpel.
Parenchymal transection
We commence by progressively opening the liver at the portal stump, which serves as a landmark for our line of transection, with an advanced energy device.
Hemostasis is performed with ligation of structures encountered along the transection plane, according to their size. Minor structures are usually treated with bipolar coagulation and the application of clips. We recommend using the Radiofrequency-assisted Habib Laparoscopic Sealer 4X, allowing a more bloodless and accurate parenchymal transection to be performed. The surgeon should be cautioned that large liver veins will be encountered in certain planes during transection.
Finally, we use a laparoscopic linear stapler device for the hepatic vein branches division and to finalize the parenchymal transection. After the transection line is near completion and hemostasis is maintained, the remaining liver parenchyma can be divided with application of repeated linear vascular staplers. Finally, the specimen is placed in a plastic bag and extracted through a separate incision, usually along a new suprapubic horizontal incision.
Patient characteristics
Between January 2012 and March 2015, sixty-two patients (41 men and 21 women) with lesions of the left lateral segment underwent laparoscopic left lateral segmentectomy using the reported resection technique. The median age of the patients was 64 years (range 42-85 years) Table 1. Outlines patient and pathology characteristics of the operated patients.
Patient Characteristics |
Number (N=64) |
Median Age (range, years) |
64 (42-85) |
Male/Female (number) |
41/21 |
Background Liver Status |
|
Normal/Chronic Hepatitis/Cirrhosis |
24/6/15 |
Median tumor number (range) |
1.73 (1-7) |
Median size of largest tumor (mm) (range) |
35.1 (8-74) |
Table 1 Baseline characteristics of HCC patients (n = 64).
Surgical outcomes
The indications for resection included primary HCC and colonic metastases. Overall the mean operative time was 172.5 min (range 40-365 min, P< 0.001). All the patients had adequate exposure of the dissection field with blood loss of mean 153 ml (range 10-770 ml, P< 0.001). None of the patients undergoing resection required any transfusion (0.0±0.0 mL vs. 200.0±109.0 mL, P< 0.001). Postoperative ascites occurred in only three of the patients and they all resolved with symptomatic treatment with albumin supplementation and diuretics within 8.5±1.6 days (P<0.001). No limitations, requiring conversions to neither laparotomy, nor intra-operative complications were encountered. The surgical outcomes and postoperative follow-up are summarized in Table 2.
Surgical Outcomes and Follow-Up of Patients |
Number (N=64) |
P-Value |
Mean Operative time (min) |
172.5 (40-365) |
P < 0.001 |
Mean blood loss (ml) (range) |
153 (10-770 ml) |
P < 0.001 |
Blood transfusion ml |
0.0 ± 0.0 ml |
P < 0.001 |
Mean Follow-up |
18.0 ± 3.6 month |
|
Cumulative Intrahepatic Recurrence Rates for Survivors |
Number (N=64) |
P-Value |
At 6 months |
8.00% |
P = 1.000 |
At 12 months |
12% |
P = 0.763 |
At 18 months |
24.60% |
P = 0.786 |
Overall complication rate |
24.50% |
P = 0.763 |
Death |
0 |
|
Wound infection |
2 |
|
Pleural effusion |
3 |
|
Chest infection |
1 |
|
Bile leak |
1 |
|
Abdominal abscess |
0 |
|
Postoperative bleeding |
1 |
|
Persistent ascites |
1 |
|
Hyperbilirubinemia |
1 |
Table 2 Surgical outcomes of patients.
Further follow-up and survival outcomes
The patients were followed up for a mean 18.0±3.6 month, with none lost to follow-up through the writing of this article. The cumulative intrahepatic recurrence rates for survivors were 8.0% (P= 1.000) at six months, 12.0% (P= 0.763) at 12 months, and 24.6% (P= 0.786) at 18 months. Intrahepatic recurrences were controlled using transcatheter arterial chemoembolization, with or without percutaneous radiofrequency ablation. The six and 18 months overall survival rates were 41.5% (P= 1.000) and 34.1% (P= 1.000), and the six and 18 months disease-free survival rates were 72.0% vs. 64.0% (P= 1.000).
During the last few decades the implementation of minimal invasive surgery in hepatic resections has evolved significantly. The approach has shifted form limited biopsies and wedge resections to highly demanding procedures like segmental resections, while abiding to oncologic principles for malignant resection. The gained experience has dramatically decreased morbidity and mortality previously associated with liver surgery. Although other modalities in treatment are available, surgical resection maintains the only means of treatment with the highest curative potential. This dramatic improvement in laparoscopic surgery makes it a viable treatment option for many patients with malignant or benign liver disease, including left lateral segmentectomy.
In patients with malignant disease, complete resection necessities a negative resection margin; a positive margin is considered a predictor of recurrence and poor survival.17 Initially 1cm of normal parenchymal margin was considered essential,20,21 however recent studies have shown that a histologic margin of a few milli meters might be adequate.15 Anatomically based segmental resections are the best means of achieving a negative margin. Non-anatomic resections predispose an increased incidence of positive margins and are associated with greater blood loss. There is relatively less surgical trauma and improved post-operative recovery, with limited need for administering plasma derivatives. It is also much simpler to re-operate in the event of tumor recurrence. The selective division of the vascular pedicles of the hepatic sector adjacent to the segment to be removed, as described in our technique, limits damage to the parenchyma caused by the en bloc clamping of the hepatic pedicle at the hilum. This advantage appears even greater in livers affected by chronic diseases.
At the hepatic hilum it is possible to identify the arterial and portal branches of the left lateral segments, 2 and 3, (anatomical left lobe), of segment 4, of the right paramedian segments, 5 and 8, and of the right lateral sector segments, 6 and 7. However also by the left extra parenchyma I route it is possible to also identify the individual segmentary branches of segments 2 and 3, which are enclosed by a sheath of connective tissue, to the left of the umbilical fissure. There are constant landmarks present on the outer surface of the liver which identify these intra-hepatic vascular structures. These landmarks make it possible to identify the margins of the segments and can be a useful guide in performing segmentary hepatectomies, in this approach a vital landmark is the U/P (umbilical/posterior) point which is used to easily identify the portal pedicles of segment 2 and 3 extra-parenchymally.
Previous publications on the extra-glissonian approach agree on the standardization of this approach.21‒23 In particular the approaches adopted by Cho et al.23 and Machado et al.22 have reported the glissonian transection. Differing in this presented study is that we avoided all the steps of encircling or occluding the pedicle throughout the procedure.
The authors' principle in this approach of laparoscopic left lateral hepatectomy follows the same principle of the open counterpart. Advantages that the authors' have seen are, ease of identification of all these structures, particularly their easy branching visibility by the scope, decrease in operative bleeding by better controlled visualization and avoiding the unnecessary resection of the U/P point. By selective occlusion of the third-order branches early in the operation by an extra-parenchymal resection provides a heightened oncologic benefit in limiting tumor cells’ spread from these segments via the portal vein.
The Glissonean pedicle transection method is a feasible, effective, and safe technique for hepatic inflow control during the curative resection of patients with large nodular HCCs. This method shortened the duration of ischemia and reduced the extent of liver parenchyme subjected to ischemia-reperfusion injury, compared with the conventional Pringle maneuver. The Glissonean method also reduced the volume of intraoperative blood loss, enhancing postoperative recovery of liver function. As an anatomical form of hepatectomy, the Glissonean method may minimize the occurrence of intrahepatic metastases, contributing to patient survival. A long-time survival follow-up study is ongoing in our institution to determine the effects on oncologic safety and survival of the Glissonean pedicle transection method in patients with large HCCs with complicating liver conditions who undergo major hepatectomy.
It utilizes the benefits of the laparoscopic approach, namely the technological advantage in clarity of the operative field while maintaining the steps of the conventional open procedure, which is technically easier. This approach when applied could serve to enhance the benefits of the oncological principles behind the left lateral segmentectomy.
None.
Author declares that there is no conflict of interest.

©2017 Gareer, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.