Journal of
eISSN: 2471-1381


Research Article Volume 2 Issue 3
1Department of Internal Medicine, Al-Azhar University, Cairo, Egypt
2Department of Microbiology and Immunology, Zagazig University, Egypt
Correspondence: Fathy Elghamry, Department of internal medicine, Al-Azhar University, Cairo 11651, Egypt
Received: September 17, 2016 | Published: October 25, 2016
Citation: Elghamry F, Alboraie M, Salim M, et al. Egy-score can predict portal hypertension and oesophageal varices in chronic hepatitis c with good sensitivity, specificity and diagnostic accuracy. J Liver Res Disord Ther. 2016;2(3):83-89. DOI: 10.15406/jlrdt.2016.02.00029
Aim: To test the ability of Egy-Score as a predictor for the presence of portal hypertension (PHT) and oesophageal varices in chronic hepatitis C (CHC).
Methods: Treatment naive CHC patients were enrolled. They were classified into 2groups according to presence or absence of PHT. Patients were subjected to liver function tests (LFTs), hepatitis C virus antibody (HCV-Ab), hepatitis B surface antigen (HBs-Ag), anti-nuclear antibody (ANA), Bilharzial agglutination titer, ultrasound abdomen and oesophago-gastro-duodensocpy. Egy-Score, AST-to-Platelet ration index (APRI) and FIB-4 were calculated according to their original formulas.
Results: Our study included 50 patients (26 males). Bilharziasis was detected in 13 patients (26%), PHT in 26 patients (52%), oesophageal varices (OV) in 23 patients (46%). Egy-Score was able todetect PHT and OV with AUROC of 0.929 and 0.890 respectively. FIB-4 was able todetect PHT and OV with AUROC of 0.798 and 0.750 respectively. APRI was able todetect PHTand OV with AUROC of 0.707 and 0.657. Bilharziasis had noimpact on the predictive ability of different scores.
Conclusion: Serum fibrosis biomarkers can predict presence of PHT and OV with good accuracy. Egy-Score was the best predictor among the studied biomarkers panels.
Keywords: egy-score, hepatitis c, portal hypertension, fibrosis, cirrhosis
PHT, portal hypertension; CHC, chronic hepatitis C; LFTs, liver function tests; HCV-Ab, hepatitis C virus antibody; HBs-Ag, hepatitis B surface antigen; ANA, anti-nuclear antibody; OV, oesophageal varices; HVPG, hepatic venous pressure gradient; APRI, aspartate aminotransferase-to-platelet ratio index; INR, international normalized ratio; AUC, under the curve; AUC, Area under the curve
Liver fibrosis and cirrhosis occurs in response to almost all causes of chronic liver injury. Increased portal pressure is the main factor determining the clinical course of cirrhosis.1 Portal Hypertension (PHT) is associated with the most severe complications of cirrhosis, such as ascites, hepatic encephalopathy and bleeding from Oesophageal Varices (OV).2 Liver biopsy and Hepatic Venous Pressure Gradient (HVPG) measurement are considered the gold standards for estimation of hepatic fibrosis and portal hypertension respectivelywithboth diagnostic and prognostic abilities.1 HVPG value above 5mmHg is suggestive of portal hypertension. An increased portal vein diameter (more than 13mm) was proposed by some authors as an independent predictor of esophageal varices.3 However, the evaluation of HVPG is an invasive procedure, which is limited to highly specialized centers and experienced operators.2 Liver biopsy is also having some disadvantages are large sampling error, consistent inter-observer disagreement, high emotional cost of patient and enormous health care commitment in case of rare but possible severe complications.4 Both methods are invasive and as such cannot be used repeatedly in clinical practice.1
Endoscopic screening is the best practice to detect the presence of varices and should be routinely used for prognostic and therapeutic indications.3 The possibility of identifying cirrhotic patients with esophageal varices or presence of other collateral by non-invasive means is appealing, in that it could decrease the necessity of endoscopic screening with reduced healthcare costs.5 At present, no satisfactory non-invasive tools are available for diagnosing clinically relevant portal hypertension and presence of varices, additional research to improve non-invasive diagnosis of varices and portal hypertension is desirable.3 The ideal non-invasive test should be safe, easy to perform, inexpensive, reproducible as well as to give numerical and accurate results in real time. It should be predictive of long term outcomes related with fibrosis and PHT to allow prognostic stratification.1 Several studies addressing this issue have been performed with varying success rates. They have either been based on laboratory parameters, i.e. platelets count or ultra sonographic features of which the spleen longitudinal diameter seems to be the most interesting one, determination of serum ammonia level, splenic, portal vein and splenic vein diameters are considered as good predictors for the presence of porto-systemic shunts in patients with liver cirrhosis.5 The capacity of ultrasound (US) to detect PHT in compensated patients is debatable. Signs of PHT such as splenomegaly, porto-systemic collaterals, portal vein diameter or portal vein velocity have great specificity but very low sensitivity especially in compensated patients. However, Doppler-US remains a key technique in patients with chronic liver disease.6 There are little data about the usefulness of serum markers in diagnosis of clinically significant PHT by comparing to HVPG. Platelet count, prothrombin index and albumin proved to correlate well with HVPG and presence of OV. However, these are the most common variables used for evaluation of cirrhosis. Among many fibrosis scores, only FibroTest® has beenstudiedas a diagnosis method in clinically significant PHT with acceptable performance. Other serum scores, like AST/ALT index, aspartate Amino Transferase-to-platelet Ratio Index (APRI), Lok or FIB-4, were correlated with presence of OV but the performances are rather sub-optimal (AUROC between 0.62 and 0.81).6‒8 Elghamry F et al.9 showed the usefulness of a new non-patented panel of biomarkers (Egy-Score) as a predictor for severe hepatic fibrosis in different chronic liver diseases. Egy-Score showed superiority in the prediction of hepatic fibrosis in chronic HCV patients over APRI, FIB-4 and Forns' index in advanced hepatic fibrosis and cirrhosis.10 Prediction of presence of portal hypertension and its complications in Egyptian patients with CHC using Egy-Score and other non-invasive biomarkers panels has not yet been explored. In this study we aimed at exploring the ability of Egy-Score as a non-invasive fibrosis biomarkers panel to predict the presence of portal hypertension and oesophageal varices in Egyptian patients with CHC. Also we aimed at comparing Egy-Score with other non-invasive fibrosis biomarkers APRI and FIB-4 in prediction of portal hypertension and its complications.
Fifty treatment naïve CHC patients were prospectively recruited from outpatient clinics of Al-Hussein University hospital in Cairo. Patients were classified according to presence of portal hypertension in to two main groups: Group1: patients without portal hypertension and Group 2: patients with portal hypertension. After full history taking and full clinical examination, all patients were subjected to: routine laboratory evaluation, urine, stool, complete blood counts, renal functions, Liver Function Tests (LFTs) including coagulation profile, ALT, AST, total and direct bilirubin, albumin and total protein), cancer antigen 19-9 (CA19-9), α-2-macroglobulin, abdominal ultrasound to detect signs of PHT, Hepatitis C virus Antibody (HCV-Ab), Hepatitis B Surface Antigen (HBSAg), Antinuclear Antibody(ANA), Bilharzial agglutination titer, endoscopy and grading of esophageal varices if present]small(grade I), medium(grade II) or large(grade III), Egy-Score was calculated according to the following formula: Egy-Score= 3.52+ 0.0063 x CA 19-9 (U/ml)+ 0.0203 x age (year)+ 0.4485 x α-2-macroglobulin(g/l)+ 0.0303 x bilirubin (umol/l)-0.0048 x platelet (109/L)-0.0462 x albumin (g/l).11 APRI and FIB-4 scores were calculated for all patients according to the following formulas: APRI score = [(AST(U/L)/upper limit (normal value) AST) ×100]/number of Platelets (10⁹/l).12 FIB-4 score= [age(years)] × AST(U/L)]/[number of platelets (10⁹/L)] × ALT (U/L)½].13 Patients with other types of chronic liver diseases were excluded.
All data were collected, tabulated and statistically analyzed using SPSS 19.0 for windows (SPSS Inc., Chicago, IL, USA) & MedCalc 13 for windows (MedCalc Software bvba, Ostend, Belgium). Quantitative data were expressed as the mean± SD & median (range) and qualitative data were expressed as absolute frequencies ''number'' & relative frequencies (percentage). Continuous data were checked for normality by using Shapiro Walk test. Independent Student t-test was used to compare two groups of normally distributed data. Mann-Whitney U was used to compare two groups of non-normally distributed data. One way ANOVA test was used to compare more than two groups of normally distributed data. Kraskall Wallis H test was used to compare more than two groups of non-normally distributed data. Percent of categorical variables were compared using Chi-square test or Fisher's exact test when appropriate. Receiver Operating Characteristic (ROC) curve analysis was used to identify optimal cut-off values for Egy-Score, APRI and FIB-4 with maximum sensitivity and specificity for prediction of portal hypertension and OV. AUROC was also calculated, criteria to qualify for AUROC were as follows: 0.90-1= excellent, 0.80-0.90= good, 0.70-0.80= fair, 0.60-0.70= poor and 0.50-0.6= fail. The optimal cutoff point was established at point of maximum accuracy. Univariate regression analysis was done to assess role of Egy-Score, APRI and FIB-4 in prediction of portal and splenic vein diameters. All tests were two sided. p< 0.05 was considered statistically significant (S), p< 0.001 was considered highly statistically significant (HS) and p≥ 0.05 was considered non statistically significant (NS).
Ethical approval and consent
The study protocol was approved by the Ethics Committee of the faculty of medicine Al-Azhar University. Data was collected anonymously with no identifying information. All participants signed informed consent for using their data in the study.
The study was carried out in the department of internal medicine, Al Hussein hospital, Al-Azhar University, Cairo, Egypt during the period between May 2015 and December 2015. Our study included fifty treatment naïve patients with CHC. They were 26 male (52%) and 24 female (48%), mean age was 55.46± 9.73 years. Group 1 (CHC patients without PHT) included 24 patients (48%) and Group 2 (CHC patients with PHT) included 26 patients (52%). Demographic, laboratory, ultrasound and endoscopic data of the studied groups are presented in (Table 1,2 and 3). Analysis of the studied scores (Egy-Score, APRI and FIB-4) in both groups showed that: Egy-Score, APRI and FIB-4 were able to predict presence of PHT however Egy-Score was superior to APRI and FIB-4. Egy-Score could predict PHT with sensitivity of 84.6%, specificity of 95.8%, Positive Predictive Value (PPV) of 95.7%, Negative Predictive Value (NPV) of 85.2% and overall accuracy of 90%. The best cut off value of Egy-Score to predict portal hypertension was >5.0955. The best cut off value of APRI to predict portal hypertension was >1.07 with sensitivity of 80.7%, specificity of 66.6%, PPV of 72.4%, NPV of 76.2% and overall accuracy of 73.9%. The best cut off value of FIB-4 to predict portal hypertension was >2.83 with sensitivity of 92.3%, specificity of 62.5%, PPV of 72.7% and NPV of 88.2% and overall accuracy of 78%. Bilharziasis had no impact on the predictive ability of different scores (Table 4) and (Figure 1).
Demographic data |
Total |
CHC patients |
Test |
p-value (Sig.) |
|||
|---|---|---|---|---|---|---|---|
Without PTH (N=24) |
With PTH (N=26) |
||||||
No. |
% |
No. |
% |
||||
Age (years) |
|||||||
Mean± SD |
53.12±10.09 |
57.61±9.06 |
-1.658 |
0.104 (NS) |
|||
Median(range) |
55(32-73) |
57(35-75) |
|||||
Sex |
|||||||
Male |
26 |
12 |
46.20% |
14 |
53.80% |
0.074 |
0.786 (NS) |
Female |
24 |
12 |
50% |
12 |
50% |
||
Bilharziasis |
|||||||
Absent |
35 |
21 |
60% |
14 |
40% |
6.731 |
0.009 (S) |
Present |
15 |
3 |
20% |
12 |
80% |
||
Table 1 Demographic data of the studied groups
Sig.: Significance, NS: Not Significant, S: Significant.
Laboratory data |
CHC patients |
Test |
p-value (Sig.) |
||
|---|---|---|---|---|---|
Without PTH (N=24) |
With PTH (N=26) |
||||
WBCs (x103/mm3) |
Mean± SD |
6.44±2.06 |
4.78±2.05 |
2.863 |
0.006 (S) |
Median(Range) |
6.65(2.6-10.6) |
4.35(1.2-10.1) |
|||
Hb(gm/dl) |
Mean± SD |
13.03±1.88 |
10.55±2.1 |
-3.955 |
<0.001 (HS) |
Median(Range) |
13.2(7-15.8) |
10.45(6.9-14.9) |
|||
Platelet count (x103/mm3) |
Mean± SD |
181±80.16 |
97.94±58.45 |
-3.972 |
<0.001 (HS) |
Median(Range) |
178(44-322) |
85.5(39-316.4) |
|||
ALT(U/L) |
Mean± SD |
55.25±31.32 |
46.74±29.13 |
-0.972 |
0.331 (NS) |
Median(Range) |
44.5(14-152) |
43(13.6-138) |
|||
AST(U/L) |
Mean± SD |
58.25±29.24 |
56.29±21.91 |
0.269 |
0.789 (NS) |
Median(Range) |
50.5(16-135) |
48(19.4-103) |
|||
Total serum bilirubin (μmol/L) |
Mean± SD |
13.99±5.44 |
24.84±12.77 |
-3.648 |
<0.001 (HS) |
Median(Range) |
12.15(5.8-32.5) |
21.7(5.1-53.5) |
|||
Direct serum bilirubin (μmol/L) |
Mean± SD |
4.33±3.01 |
11.13±8.85 |
-3.119 |
0.002 (S) |
Median(Range) |
3.4(1.7-13.7) |
8.7(1-31.1) |
|||
Total protein(gm/dl) |
Mean± SD |
7.77±0.4 |
7.25±0.78 |
-3.458 |
0.001 (S) |
Median(Range) |
7.9(7-8.5) |
7.5(3.9-8) |
|||
Albumin(gm/L) |
Mean± SD |
41.15±4.56 |
28.9±7.87 |
-5.231 |
<0.001 (HS) |
Median(Range) |
40.5(35-57) |
28.5(13-40) |
|||
PT(sec.) |
Mean± SD |
13.12±0.97 |
15.5±2.33 |
-4.638 |
<0.001 (HS) |
Median(Range) |
13(11.2-15.2) |
15.2(11.9-21) |
|||
PTT(sec.) |
Mean± SD |
32.24±3.8 |
36.64±4.26 |
-2.728 |
0.006 (S) |
Median(Range) |
30.5(27-39) |
36.65(26.1-41) |
|||
INR |
Mean± SD |
1.07±0.08 |
1.37±0.49 |
-4.271 |
<0.001 (HS) |
Median(Range) |
1.04(1-1.29) |
1.21(1-3.6) |
|||
Serum creatinine (mg/dl) |
Mean± SD |
0.87±0.19 |
0.94±0.28 |
-0.772 |
0.44 (NS) |
Median(Range) |
0.8(0.6-1.3) |
0.9(0.56-1.6) |
|||
α2 macroglobulin(gm/L) |
Mean± SD |
3.93±0.51 |
3.81±0.47 |
0.836 |
0.407 (NS) |
Median(Range) |
3.9(3.2-5.3) |
3.8(3.1-4.7) |
|||
CA19-9 (U/ml) |
Mean± SD |
51.27±42.28 |
114.23±106.77 |
-1.99 |
0.047 (S) |
Median(Range) |
48.6(0-186) |
76(3.3-317.9) |
|||
Table 2 Laboratory data of the studied groups
Ultra sonographic & endoscopic findings |
Total |
CHC patients |
Test |
p-value (Sig.) |
|||
|---|---|---|---|---|---|---|---|
Without PTH (N=24) |
With PTH (N=26) |
||||||
No. |
% |
No. |
% |
||||
Portal vein diameter (mm) |
|||||||
Mean± SD |
10.81±1.10 |
15.45±2.52 |
-5.543 |
<0.001 (HS) |
|||
Median(range) |
11 (9-13) |
15.70(10-23) |
|||||
Splenic diameter (mm) |
|||||||
Mean± SD |
120.70±23.83 |
176.01±29.41 |
-7.267 |
<0.001 (HS) |
|||
Median(range) |
110(86-170) |
177(120-230) |
|||||
Ascites |
|||||||
Absent |
31 |
24 |
100% |
7 |
26.90% |
28.288 |
<0.001 (HS) |
Mild |
5 |
0 |
0% |
5 |
19.20% |
||
Moderate |
6 |
0 |
0% |
6 |
23.10% |
||
Marked |
8 |
0 |
0% |
8 |
30.80% |
||
OV |
|||||||
Absent |
27 |
24 |
100% |
3 |
11.50% |
39.316 |
<0.001 (HS) |
Grade I |
11 |
0 |
0% |
11 |
42.30% |
||
Grade II |
7 |
0 |
0% |
7 |
26.90% |
||
Grade III |
5 |
0 |
0% |
5 |
19.20% |
||
Table 3 Ultra sonographic & endoscopic findings of the studied groups
Variables |
CHC patients |
Test |
p-value (Sig.) |
|||
|---|---|---|---|---|---|---|
Without PTH (N=24) |
With PTH (N=26) |
|||||
Without Bilharziasis (N=21) |
With Bilharziasis (N=3) |
Without Bilharziasis (N=14) |
With Bilharziasis (N=12) |
|||
Egy-Score |
||||||
Mean± SD |
4.32±0.66 |
4.40±0.59 |
5.91±1.09 |
6.25±1.07 |
15.434 |
<0.001 (HS) |
Median(range) |
4.47(2.81-5.57) |
4.08(4.04-5.10) |
5.78(3.95-7.78) |
5.81(5-8.09) |
||
Test |
-0.189 |
-0.793 |
||||
p-value |
0.582 |
0.435 |
||||
(Sig.) |
(NS) |
(NS) |
||||
APRI |
||||||
Mean± SD |
1.08±0.92 |
1.10±0.82 |
1.48± 0.77 |
1.98± 0.97 |
7.365 |
0.061 (NS) |
Median(range) |
0.663(0.21-3.62) |
0.976(0.33-1.98) |
1.41(0.42-3.40) |
1.47(0.56-3.60) |
||
Test |
-0.218 |
-1.453 |
||||
p-value |
0.827 |
0.159 |
||||
(Sig.) |
(NS) |
(NS) |
||||
FIB-4 |
||||||
Mean± SD |
3.10±2.35 |
3.41± 3.01 |
5.36± 2.48 |
6.79± 3.19 |
13.906 |
0.003 (S) |
Median(range) |
2.34(0.63-8.76) |
2.3(1.11-6.82) |
5.3(1.25-9.23) |
6.23(2.92-13.89) |
||
Test |
-0.131 |
-1.029 |
||||
p-value |
0.896 |
0.304 |
||||
(Sig.) |
(NS) |
(NS) |
||||
Table 4 Comparison between CHC patients in both groups without and with bilharziasis as regard Egy-Score, APRI and FIB-4.
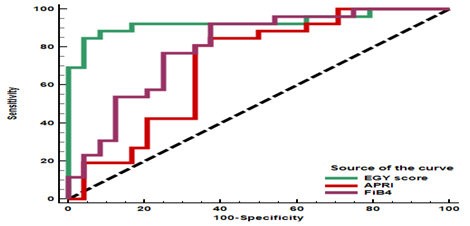
Figure 1 Receiver Operating Characteristic (ROC) curve of EGY-score, APRI and FIB-4 as a predictor for portal hypertension in chronic hepatitis patients.
Egy-Score, APRI and FIB-4 were able to predict presence of OV however Egy-Score was superior to APRI and FIB-4. Only Egy-Score and FIB-4 were able to predict different grades of esophageal varices. Egy-Score could predict OV with sensitivity of 88.8%, specificity of 86.9%, PPV of 87%, NPV of 88.9% and overall accuracy of 87.9%. The best cut off value of Egy-Score to predict OV was >5.0955. The best cut off value of APRI to predict OV was >0.976 with sensitivity of 82.6%, specificity of 55.5%, PPV of 61.3%, NPV of 78.9% and overall accuracy of 68%. The best cut off value of FIB-4 to predict OV was >2.83 with sensitivity of 91.3%, specificity of 55.5%, PPV of 63.6%, NPV of 88.2% and overall accuracy of 72% (Table 5 and 6) and (Figure 2).
Variables |
CHC patients |
Test |
p-value (Sig.) |
|
|---|---|---|---|---|
No OV (N=27) |
OV (N=23) |
|||
Egy-Score |
||||
Mean± SD |
4.51± 0.84 |
6.09± 1.09 |
-5.759 |
<0.001 (HS) |
Median (range) |
4.55 (2.81-7.22) |
5.80 (3.95-8.09) |
||
APRI |
||||
Mean± SD |
1.17± 0.88 |
1.69± 0.94 |
-1.898 |
0.058 (NS) |
Median (range) |
0.96 (0.21-3.62) |
1.44 (0.42-3.60) |
||
FIB-4 |
||||
Mean± SD |
3.49± 2.52 |
5.98± 2.98 |
-3.017 |
0.003 (S) |
Median(range) |
2.41 (0.63-8.76) |
5.6 (1.25-13.89) |
||
Table 5 Comparison between CHC patients without OV and with OV as regard Egy-Score, APRI and FIB-4
Variables |
CHC patients |
Test |
p-value (Sig.) |
|||
|---|---|---|---|---|---|---|
Absent OV (N=27) |
Grade I OV (N=11) |
Grade II OV (N=7) |
Grade III OV (N=5) |
|||
Egy-Score |
||||||
Mean± SD |
4.51±0.84 |
6.18±1.15 |
6.10±0.85 |
5.85±1.41 |
10.822 |
<0.001 (HS) |
Median(range) |
4.55(2.81-7.22) |
5.8(4.18-8.09) |
5.75(5.35-7.49) |
5.88(3.95-7.23) |
||
APRI |
||||||
Mean± SD |
1.17±0.88 |
2.02±1.02 |
1.39±0.55 |
1.39±1.09 |
5.989 |
0.112 (NS) |
Median(range) |
0.96(0.21-3.62) |
1.87(0.45-3.60) |
1.35(0.74-2.55) |
1.33(0.42-3.18) |
||
FIB-4 |
||||||
Mean± SD |
3.49± 2.52 |
7.27± 3.38 |
5.20±1.45 |
4.22±2.78 |
11.867 |
0.008 (S) |
Median(range) |
2.41(0.63-8.76) |
6.29(2.29-13.89) |
4.83(3.37-7.35) |
3.19(1.25-8.51) |
||
Table 6 Relation between OV and Egy-Score, APRI, FIB-4 in CHC patients
Portal hypertension and oesophageal varices are main complications of chronic liver disease and may lead to deathespecially in patients with cirrhosis. The presence and the degree of portal hypertension should be evaluated in all patients with cirrhosis and other chronic liver diseases.14 The degree of portal hypertension can be correlated with the severity of cirrhosis, which is estimated by the Child-Pugh score.15 Increased hepatic vascular resistance in patients with cirrhosis is also influenced by the presence and extent of fibrosis.16 In one recent study, the area of liver collagen, which is the major component of fibrous tissue, was measured by computer-assisted image analysis and was found to be significantly correlated with the HVPG in patients with cirrhosis.17 Accordingly, an evaluation of the extent of hepatic fibrosis may provide information about the presence and severity of portal hypertension.The noninvasive estimation of hepatic fibrosis has been a subject of extensive research in the last 10years. However, only a few of these procedures have been evaluated for the noninvasive diagnosis of gastroesophageal varices.18
Egy-Score is an efficient, cost-effective and promising score in the prediction and diagnosis of significant and advanced fibrosis and cirrhosis with good sensitivity and specificity.11 The Egy-Score showed superiority over the APRI, FIB-4 and Forns’ index for detecting advanced hepatic fibrosis and cirrhosis, while Forns’ index was superior to the Egy-Score, APRI and FIB-4 in detecting significant fibrosis.10 In this study we aimed at exploring the ability of Egy-Score, APRI and FIB-4as non-invasive fibrosis biomarkers panels to predict the presence of portal hypertension and oesophageal varices in Egyptian patients with CHC. The aim of our study is concordant with Calvaruso V et al.17 who concluded that an evaluation of the extent of hepatic fibrosis may provide information about the presence and severity of portal hypertension.Our results showed a highly significant positive correlation between portal hypertension and Egy-Score, FIB-4, portal vein diameter, splenic diameter, OV, hemoglobin, Platelet count, serum albumin, PT, INR and total bilirubin. We found significant correlation between portal hypertension and bilharziasis, CA19-9, PTT and direct bilirubin. On the other hand portal hypertension was negatively correlated to age, sex, ALT, AST, A-2-M and serum creatinine. These findings are concordant with results of Sarwar S et al.20 who found that patients with low serum albumin levels, which were associated with low platelet counts and large portal vein diameters, were more likely to have severe portal hypertension and varices. These findings also are concordant with results of De Bruyn G et al.21 who reported that splenomegaly, spider nevi, presence of abdominal wall collateral circulation are highly specific for portal hypertension. Berzigotti A et al.22 found that Ultrasonography (US) is the first-line imaging technique recommended for the diagnosis and follow-up of patients with portal hypertension, since it is non-invasive, cheap and can be performed at bedside. US is highly specific for the diagnosis of cirrhosis and portal hypertension, but its sensitivity is relatively low in compensated patients. Splenomegaly is the most common and sensitive sign of portal hypertension. It is an independent predictor of esophageal varices and is a marker of clinical significant portal hypertension (CSPH) in compensated cirrhotic patients.
Progressive spleen enlargement has been shown to predict variceal formation and growth and is associated with a higher probability of clinical decompensation. Analysis of the Egy-Score, APRI and FIB-4 in the studied groups showed that: Egy-Score, APRI and FIB-4 were able to predict presence of PHT and oesophageal varices. These findings support the results of Abraldes JG et al.23 who found that surrogate biomarkers(Albumin, bilirubin, international normalized ratio (INR) or their combination in the Child-Pugh score correlate with HVPG and portal hypertension and Berzigotti A et al.22 who found that a model combining albumin, ALT and INR had an area under the curve (AUC) of 0.952 in predicting clinically significant portal hypertension and Verma V et al.25 who reported that APRI score ≥1.09seems to have an acceptable accuracy for prediction of high portal pressure. Abraldes JG et al.23 also found that Albumin, bilirubin, international normalized ratio (INR) or their combination in the Child-Pugh score correlate with the prevalence and grade of esophageal varices in cirrhotic patients, and Zaman A et al.26 have reported that thrombocytopenia was the single laboratory test most frequently associated with the presence of varices and of large esophageal varices.
Sharma SK et al.27 also found that Splenomegaly and platelet count were the independent predictors for the presence of large varices and Mandal L et al.28 reported that patients with gastro esophageal varices, grading of varices directly correlated with spleen size. That implied, when spleen size increased, gastro-esophageal varices also transformed to higher grades. Egy-Score provides information for all patients and can easily be applied to clinical routine. Testing for CA 19-9, platelet count, total bilirubin and albumin could be done in most hospitals and laboratories. α-2-macroglobulin is available to any laboratory with a nephelometer. It is therefore less expensive and more convenient to apply Egy-Score if a noninvasive method for prediction of portal hypertension or oesophageal varices is needed rather than using expensive invasive and difficult tests.
Our study was cross sectional and exploratory so our results should be validated in other large scale of patients with liver disease of distinct etiology. Our study included only one type of chronic liver disease (CHC) so our results should be validated in other types of chronic liver diseases. CA19-9 and α-2-macroglobulin should be performed to calculate Egy-Score which are not measured routinely in assessment of chronic HCV patients compared to APRI and FIB-4.
Egy-Score shows a highly significant correlation and a very good prediction to portal hypertension and oesophageal varices. Egy-Scorewas superior to APRI and FIB-4 for prediction of portal hypertension and oesophageal varices. Egy-Score shows NPV 88.9% in detection of OV so we can use Egy-Score to differentiate between patients who need to do upper endoscopy from patients who should not do upper endoscopy to detect OV.
None.
Author declares that there is no conflict of interest.

©2016 Elghamry, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.