Journal of
eISSN: 2471-1381


Research Article Volume 2 Issue 2
1Department of Nutritional Science, Morioka University, Japan
2Department of Internal Medicine, Iwate Medical University, Japan
3Department of Internal Medicine, Morioka City Hospital, Japan
Correspondence: Kazuyuki Suzuki, Department of Nutritional Science, Morioka University, Sunakomi 808, Takizawa 020-694, Iwate, Japan, Tel 8119 6885 555, Fax 8119688 5577
Received: May 30, 2016 | Published: August 11, 2016
Citation: Suzuki K, Onodera M, Hidekatsu K, et al. Reevaluation of serum carnitine status in patients with liver cirrhosis. J Liver Res Disord Ther. 2016;2(2):58-64. DOI: 10.15406/jlrdt.2016.02.00025
Background: Carnitine (CA) is an important key substance for lipid metabolism and serum CA levels change in patients with various diseases. Although liver cirrhosis (LC) is known as a representative cause of secondary CA deficiencies, the exact levels of serum CA in LC patients remains a controversial issue. The aim of the present study was to reevaluate the serum CA levels in LC patients, with a particular focus on the relationship between serum CA levels and liver and renal function.
Methods: Seventy-one patients with LC (51 males, 20 females; median age 63.0years) were examined. Fasting serum Total-CA (T-CA), Free-CA (F-CA) and Acyl-CA (Ac-CA) levels were measured using an enzymatic cycling method and were evaluated according to their relationships with liver and renal dysfunction.
Results: The levels (mean±standard deviation, μmol/L) of serum T-CA, F-CA and Ac-CA (72.9±19.1, 55.3±14.0 and 17.6±7.2 respectively) in LC patients were almost within normal range (T-CA: 45.0-91.0; F-CA: 36.0-74.4 and Ac-CA: 6.0-23.0 respectively). Approximately 11% of patients had serum T-CA levels <45μmol/L. These serum CAs levels did not correlate with severity of liver damage based on the Child-Pugh classification and the Model for End-stage Liver Disease, but serum T-CA and Ac-CA levels significantly correlated with serum creatinine and/or the estimated glomerular filtration rate. Blood ammonia levels did not correlate with these serum CA levels. Among nutritional parameters, only serum free fatty acid levels strongly correlated with serum Ac-CA levels and the Ac-CA to T-CA ratio.
Conclusion: Our results suggest that the rate of secondary CA deficiency may be low and that serum CA levels are almost within normal range in LC patients, although serum Ac-CA levels are closely associated with renal dysfunction. Furthermore, the Ac-CA to T-CA ratio may be useful as a marker of malnutrition (299).
Keywords: carnitine, liver cirrhosis, free fatty acid, renal dysfunction, hepatic encephalopathy, malnutrition
CA, carnitine; T-CA, total carnitine; F-CA, free-carnitine; Ac-CA, acyl-carnitine; L-CA, levo-carnitine; LC, liver cirrhosis; BTR, branched-chain amino acids to tyrosine ratio; OHE, overt hepatic encephalopathy; MHE, minimal hepatic encephalopathy; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; FFA, free fatty acid; PEM, protein energy malnutrition; PT, prothrombin time; HBV, hepatitis B virus; HCV, hepatitis c virus; HBsAg, hepatitis b surface antigen; HbA1c, hemoglobin A1c; T-Bil, total bilirubin; Alb, albumin; BUN, blood urea nitrogen; CRN, creatinine; BS, blood sugar; NH3, ammonia, Zn, zinc; BCAA, branched chain amino acids; Tyr, tyrosine; MELD, model for end-stage liver disease; ALC, acetyl-l-carnitine; PT-INR, prothrombin time international ratio
Levo Carnitine (L-CA) is an important regulator of lipid metabolism. It is responsible for integrating the long-chain fatty acids into the mitochondria matrix and facilitating beta-oxidation and energy production through the Krebs cycle.1-3 In humans, 75% of carnitine (CA, β-hydroxyl-γ-trimethyl amino butyric acid) is obtained from the diet, in particular, food from animal sources and is mainly absorbed via both active and passive transport across enterocyte membranes of the intestine. After absorption, CA is synthesized from the essential amino acids lysine and methionine in the liver, kidney and brain. CA is mainly stored in the muscles, ultimately, CA and its metabolites (Free-CA and Acyl-CA) are excreted with the urine and stool.1-4 Although circulating CA and its metabolites constitute only 3% of total body CA volume, their concentrations [Total CA (T-CA), Free CA (F-CA) and Acyl CA (Ac-CA)] are altered in variations of metabolic status in human diseases such as diabetes mellitus, hemodialysis, liver injury, drug interactions, endocrine imbalance and other disorders.3 In particular, liver cirrhosis (LC) is considered to be a representative secondary CA deficiency caused by inadequate food intake, decreased CA biosynthesis in the liver and decreased muscle volume.5,6 LC patients with secondary CA deficiency may often have several symptoms such as general fatigue and muscle cramp, resulting on the worse of quality of life in LC patients.3 However, the serum or plasma concentrations of CA in LC patients remains a controversial issue for several reasons.6-8 One is that a gold standard method for measuring serum or plasma CA concentrations has not been established. Another is that circulating CA concentrations are affected by renal function. Finally, the nutritional state in terms of calorie intake has been found to be different among individual LC patients worldwide.
Recently, L-CA and/or acetyl-L-CA (ALC) have been used as therapeutic options in LC patients with minimal and overt hepatic encephalopathy (MHE and OHE respectively).9-14 ALC induces urea genesis, leading to decreased blood and brain ammonia levels, although L-CA administration is thought to improve ammonia metabolism via energy metabolism in rat brains with porta-systemic shunts. 10,11,15Additionally, since ALC acts as a direct antioxidant, ALC supplementation has been shown to be effective in inhibiting oxidative stress and death in rat models of dementia and chronic ischemia.16-19 However, in the clinical studies in LC patients with MHE or OHE-except for a report by Saito, et al.14 the concentrations of serum or plasma CA have not been clearly reported. In addition, it has not been fully confirmed whether the administration of L-CA or ALC is necessary in LC patients with MHE and OHE. Therefore, to establish L-CA and/or ALC therapies in the LC patients with MHE and OHE, several issues need to be resolved. The first is to reevaluate the circulating CA levels involving the rate of secondary CA deficiency in LC patients with different etiologies, the second is to clarify the relationship between CA and ammonia in metabolic organs and the third is to confirm the indication and useful dosage of these drugs administered to LC patients with MHE and OHE.
In the present study, as a first step to resolving the above issues, we reevaluated the serum CA levels and examined the relationships between serum CA status and liver and renal functions involving nutritional parameters in patients with LC.
Materials
Seventy one patients with LC visiting Iwate Medical University Hospital during the period from January 2012 to March 2015 were enrolled. The diagnosis of LC was based on routine biochemical examination including peripheral blood count, liver function tests and Prothrombin Time (PT) Activity, Ultrasonography, Computed Tomography and/or liver biopsy. The etiology of LC was decided as follows. Hepatitis B virus (HBV) and hepatitis C virus (HCV) related LC was based on positivity for hepatitis B surface antigen (HBsAg) and positivity for both anti-HCV and HCV-RNA respectively. Alcoholic LC was determined by the history of alcohol intake (both an ethanol consumption of ≥40 g/day and duration of ≥10 years). Autoimmune LC included autoimmune chronic hepatitis and primary biliary cirrhosis which was diagnosed based on the results of serum anti-nuclear antibody, anti-mitochondria antibody testing and liver pathology. LC due to nonalcoholic steatohepatitis was diagnosed in patients with fatty liver disease without HBV or HCV infections, alcoholic abuse or autoimmune liver diseases. Cryptogenic LC was defined as LC without any of the aforementioned etiologies. The presence of esophageal varices was examined by upper gastro endoscopy. Diabetes mellitus was diagnosed on the basis of elevated fasting plasma glucose levels (>126 mg/dL) or elevated hemoglobin A1c (HbA1c) (>6.5%) according to the Diagnostic Criteria for Diabetes Mellitus in Japan, or by the patient’s use of anti-diabetic agents. Hepatocellular carcinoma was diagnosed by elevated serum tumor markers (alpha-fetoprotein and/or protein induced by vitamin K absence or vitamin K antagonist-II) in conjunction with imaging studies such as ultrasonography, computed tomography and magnetic resonance imaging. The presence of these complications was judged when the above examinations were performed within 6 months before commencement of this study. Background information of the patients with LC is shown in Table 1.
Methods
Peripheral blood samples were drawn for each patient in the fasting state before breakfast when the patients visited our clinic in the morning. Biochemical examination involved peripheral blood counts (Erythrocytes, Leukocytes and Platelets), Liver Function Tests [Total-Bilirubin (T-Bil), Aspartate Aminotransferase, Alanine Aminotransferase, Albumin (Alb)], Renal Function Tests [(Blood Urea Nitrogen (BUN) and Creatinine (CRN)], Electrolytes (Sodium, Potassium and Chloride), PT activity, Blood Sugar (BS), Free Fatty Acid (FFA) and Ammonia (NH3) by standard clinical laboratory methods. Serum Zinc (Zn), Total Branched-Chain Amino Acids (BCAA: Valine, Leucine and Isoleucine) to Tyrosine (Tyr) ratio (BTR) and CA status (T-CA, F-CA, Ac-CA and Ac-Ca to T-CA ratio) were measured at the Bio Medical Laboratories Inc., Sendai, Japan (Zn using the atomic absorption method, BTR using the enzymatic method and CA using the enzymatic cycling method respectively). The severity of liver dysfunction and the prognostic score were evaluated according to the Child-Pugh classification and the Model for End-stage Liver Disease (MELD) score respectively [20, 21]. The estimated glomerular filtration rate (eGFR) was calculated and the grading of chronic kidney disease (CKD) was diagnosed according to the Japanese Kidney Association Society [22] and modified into three grades: grade 0 (within normal renal function, eGFR ≥90 ml/min/1.73m2), grade 1 (mild renal dysfunction, eGFR ≥60 ml/min/1.73m2) and grade 2 (moderate and severe renal dysfunction, eGFR <59 ml/min/1.73m2).
Statistical analysis
Data are expressed as mean± standard deviation (M±SD) and/or median (25th-75th percentiles) if the data did not show the normal distribution. Nonparametric multiple comparisons were made by the Mann-Whitney U-test. Categorical comparisons were made by Fisher’s exact test. A decision-tree algorithm was constructed, and the categorical differences in the decision-tree model were analyzed by the χ2-test. These statistical tests were performed using SPSS 12.0 software (SPSS, Chicago, IL, USA). P <0.05 was defined as a statistically significant level.
Ethics
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committees of Iwate Medical University. The study was performed after obtaining written informed consent from each patient.
Numbers |
71 |
Age (years, mean ± SD) |
62.5 ± 10.5 |
Gender (male/female) |
51/20 |
Etiology of liver cirrhosis+ |
|
HBV |
4 |
HCV |
25 |
HCV + Alcoholic |
4 |
Alcoholic |
24 |
Others++ |
14 |
Laboratory data (reference ranges*) |
|
(mg/dL)(0.2-1.1) |
1.5 ± 1.3 |
Albumin (g/dL)(4.3-5.4) |
3.6 ± 0.6 |
Prothrombin time (International ratio)(0.92-1.04) |
1.17 ± 0.24 |
Ammonia (μg/dL)(20-60) |
73 ± 42 |
Creatinine (mg/dL)(0.3-1.0) |
0.89 ± 0.46 |
eGFR (ml/min/1.73mm2) (>90)** |
72 ± 24 |
Blood sugar (mg/dL)(60-100) |
112 ± 26 |
Free fatty acid (μmol/L)(0.10-0.81) |
736 ± 387 |
Zinc (mg/dL)(59-135) |
70 ± 21 |
Branched-chain amino acid to tyrosine ratio (4.99-9.45) |
4.56 ± 2.20 |
Complications |
|
Esophageal varices (present/absent/unknown) |
47/7/17 |
Diabetes mellitus (present/absent/unknown) |
25/33/13 |
Hepatocellular carcinoma (present/absent) |
24/47 |
History of overt hepatic encephalopathy (present/absent) |
Dec-59 |
Child-Pugh classification (grade A/B/C) |
48/19/4 |
MELD score (~9/10~14/15~20 points) |
41/20/10 |
Table 1 Clinical background in LC patients
Data are expressed as mean ± standard deviation.
+ HBV, HCV, Al. ++others; involve autoimmune, nonalcoholic steatohepatitis and cryptogenic factor.
*Reference ranges of each parameter are used the values of Iwate Medical University Hospital and the Bio Medical Laboratories Inc., Sendai, Japan, **eGFR.
Serum T-CA, F-CA and Ac-CA levels in LC patients
Serum T-CA, F-CA and Ac-CA levels (M±SD) in all LC patients were 72.9±19.1 μmol/L, 55.3±14.0 μmol/L and 17.6±7.2 μmol/L respectively, these levels were within normal range according to the Japanese literature (M±2SD, T-CA: 45.0-91.0 μmol/L, F-CA: 36.0-74.4 μmol/L and Ac-CA: 6.0-23.0 μmol/L respectively). The Ac-CA to T-CA ratio in serum was 0.23±0.06 in LC patients, although the range of this ratio in healthy subjects is not shown. Serum T-CA showed a positive correlation both with serum F-CA and Ac-CA (T-CA vs. F-FA: r=0.935, p<0.001; T-CA vs. Ac-CA: r=0.70, p<0.001). This correlation was more significant with serum F-CA than with serum Ac-CA. The serum Ac-CA to T-CA ratio showed strong correlation with Ac-CA levels (r=0.771, p<0.001) but no correlation with serum T-CA or F-CA levels (vs. T-CA: r=0.186, vs. F-CA: r=-0.125 respectively). In assuming a serum T-CA level of <45 μmol as secondary CA deficiency, 8(11.3%) of the total number of LC patients matched this criterion. The rate of secondary CA deficiency was not associated with the Child-Pugh classification or the MELD score (data not shown). The serum CA levels showed no significant differences between patients with hepatitis virus-related LC and alcoholic LC. Specifically, the T-CA, F-CA and Ac-CA levels were 68.7±17.7μmol/L, 52.6±12.9μmol/L and 16.1±7.3μmol/L respectively, in patients with virus-related LC (HBV+HCV, total cases 29) and 74.1±15.7μmol/L, 56.4±12.9 μmol/L and 17.7±5.7 μmol/L respectively, in patients with alcoholic LC (24 cases except alcoholism +HCV cases). Furthermore, among each etiology (HBV, HCV, alcoholism, alcoholism +HCV and others involving autoimmune and cryptogenic), the serum T-CA, F-CA and Ac-CA levels did not have significant differences. Additionally, the Ac-CA to T-CA ratio in serum did not show any significant difference in relationship with the etiologies of LC.
Relationship with severity of liver damage based on Child-Pugh classification
The serum T-CA, F-CA and Ac-CA levels (median [25th-75th percentiles]) were 72.8[63.6-84.7] μmol/L, 57.4[47.3-61.4] μmol/L and 15.7[13.0-22.5] μmol/L in Child-Pugh grade A, 63.4[58.6-70.0] μmol/L, 48.8[44.7-53.5] μmol/L and 13.6[11.6-16.6] μmol/L in Child-Pugh grade B and 106.7[91.1-114.5] μmol/L, 78.6[66.5-85.3] μmol/L and 28.1[22.1-31.7] μmol/L in Child-Pugh grade C. The Ac-CA to T-CA ratio also showed no significant differences in relationship with the Child-Pugh [grade A: 0.25[0.20-0.28]; grade B: 0.23[0.19-0.26]; and grade C: 0.26[0.22-0.29] respectively). There were no significant relationships between serum CA levels and Child-Pugh grade except a significant difference between grades A and B in serum T-CA levels (Figure 1). Furthermore, there was no significant relationship between serum CA levels and the MELD scores (data not shown).
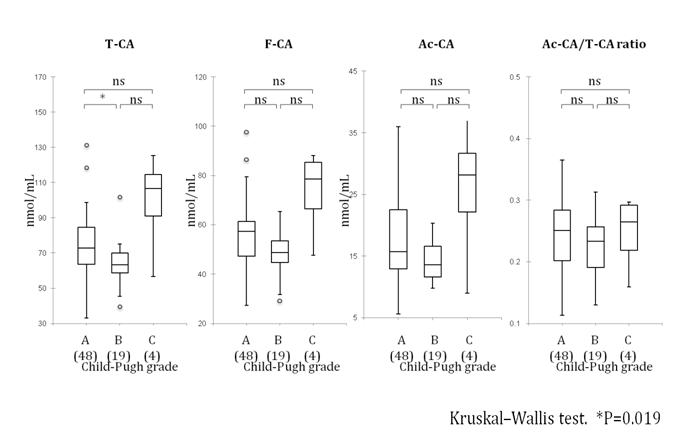
Figure 1 Relationship between serum carnitine levels and Child-Pugh class
Data are expressed as median [25th-75th percentiles]. Statistical analysis carried out using the Kruskal-Wallis test. *p<0.05; ns: not significant; A: class A; B: class B; C: class C based on Child-Pugh classification.
( ): the number of patients.
Correlation between serum CA levels and biochemical parameters
Serum T-CA levels did not correlate with any parameters except eGFR. Serum F-CA levels did not correlate with any parameters, while serum Ac-CA levels showed significant correlation with the levels of serum FFA, CRN and eGFR. The serum Ac-CA to T-CA ratio showed significant correlation with the BS and FFA levels but not with serum CRN and eGFR (Table 2).
Relationship between serum CA levels and renal functions
Serum T-CA, F-CA and Ac-CA levels among each grade of eGFR were 69.7[56.9-71.9] μmol/L, 52.9[44.3-57.9] μmol/L and 14.0[11.3-17.2] μmol/L in grade 0, 72.1[63.1-85.6] μmol/L, 54.6[46.8-61.5] μmol/L and 15.5[12.6-22.7] μmol/L in grade 1 and 76.7[62.9-90.0] μmol/L, 58.3[48.9-64.7] μmol/L and 17.7[13.8-23.9] μmol/L in grade 2. The Ac-CA to T-CA ratios were 0.23[0.17-0.25] in grade 0, 0.26[0.18-0.29] in grade 1 and 0.25[0.22-0.27] in grade 2. The serum CA levels and the Ac-CA to T-CA ratio showed no significant differences among the eGFR grades (Figure 2).
However, the relationships among the serum CA levels and the Ac-CA to T-CA ratio and eGFR in each patient showed significant correlation (Figure 3). Additionally, we examined the relationship between serum CRN levels and eGFR in LC patients. Serum CRN levels in LC patients are often underestimated, because CRN is synthesized in the muscle and its serum level has been strongly affected by the volume of muscle in LC patients who have evidence of malnutrition (protein-energy malnutrition, PEM) and/or sarcopenia. Generally, in chronic kidney disease, it has been considered that an eGFR level of 50 ml/min/1.73mm2 has been approximately equal to a serum CRN level of 2mg/dl.22 As shown in Figure 4, the correlation between levels of serum CRN and eGFR in LC patients was significantly negative statistically and eGFR of 50 ml/min/1.73mm2 might be equal to serum CRN of 1.22 mg/dl.
Parameters+ |
T-CA |
F-CA |
Ac-CA |
Ac-CA/T-CA ratio |
||||
|---|---|---|---|---|---|---|---|---|
r |
P |
r |
P |
r |
P |
r |
P |
|
T-Bil |
0.044 |
n.s. |
0.019 |
n.s. |
0.153 |
n.s. |
0.12 |
n.s. |
Alb |
0.044 |
n.s. |
-0.043 |
n.s. |
0.159 |
n.s. |
0.186 |
n.s. |
PT-INR |
0.005 |
n.s. |
0.115 |
n.s. |
0.088 |
n.s. |
-0.225 |
n.s. |
BTR |
-0.069 |
n.s. |
-0.075 |
n.s. |
-0.135 |
n.s. |
-0.058 |
n.s. |
Zn |
0.154 |
n.s. |
0.104 |
n.s. |
0.11 |
n.s. |
0.111 |
n.s. |
FAA |
0.164 |
n.s. |
-0.033 |
n.s. |
0.59 |
<0.001 |
0.659 |
<0.001 |
BS |
-0.089 |
n.s. |
-0.206 |
n.s. |
0.199 |
n.s. |
0.281 |
<0.05 |
B-NH3 |
0.102 |
n.s. |
0.168 |
n.s. |
-0.052 |
n.s. |
-0.197 |
n.s. |
CRN |
0.166 |
n.s. |
0.11 |
n.s. |
0.236 |
<0.05 |
0.183 |
n.s. |
eGFR |
-0.273 |
<0.05 |
-0.208 |
n.s. |
-0.303 |
<0.01 |
-0.222 |
n.s. |
Table 2 Correlation between the levels of serum CAs and biochemical parameters
P values are based on the Spearman’s rank correlation coefficient. T-CA, F-CA, Ac-CA, T-Bil, Alb, PT-INR, BTR, Zn, FFA, BS, B-NH3, CRN and eGFR.
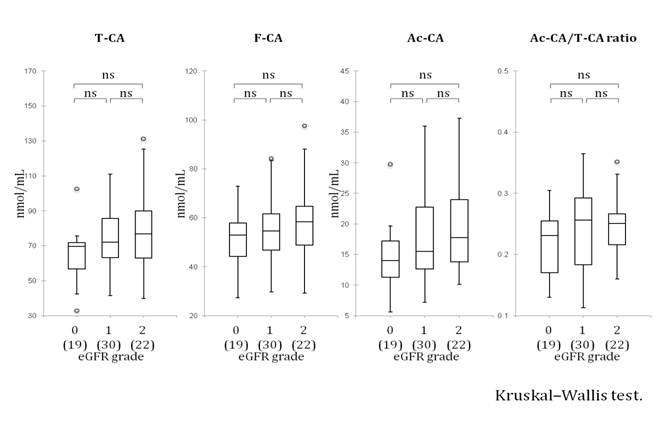
Figure 2 Relationship between serum carnitine levels and the grading of estimated glomerular filtration rate
Data are expressed as median [25th-75th percentiles]. Statistical analysis carried out using the Kruskal-Wallis test. ns: not significant; ( ): the number of patients. eGFR, which was modified and classified into three groups according to the criteria of the Japanese Kidney Association Society. Grade 0: eGFR>90 ml/min/1.73m2; grade 1: eGFR- 89-60 ml/min/1.73m2; grade 2: eGFR< 59 ml/min/1.73m2.
Figure 3 Correlation between serum carnitine levels and estimated glomerular filtration rate Statistical analysis carried out using Spearman’s rank correlation coefficient.
LC has been considered as one of the secondary CA deficiency diseases because LC patients have poor protein intake, decreased CA biosynthesis and decreased muscle volume.3,5 To appropriately assess a patient’s CA status-in particular, CA deficiency in LC-it will be necessary to measure the CA concentration in the muscle. Because such an invasive examination cannot be performed in a clinical situation, the measurement of plasma or serum CA levels is considered as the only procedure for assessing CA deficiency in LC patients. However, many previous reports concerning plasma or serum CA (T-CA, F-CA and Ac-CA) levels have been controversial.6-8 Specifically, Rudman et al.6 showed that CA deficiency is common in LC patients with advanced malnutrition,6 while another three reports showed elevated serum or plasma CA levels in LC patients.7,8 In particular, Amodio et al.7 reported that high levels of plasma CA were seen in patients independently of the etiology of LC and associated with malnutrition and alcohol abuse.7 This discrepancy is considered to be caused by the following factors:
In the present study, we showed that the serum CA levels using an enzymatic cycling method the patients with LC were almost within the Japanese baseline range and approximately 11% of LC patients had serum T-CA levels of <45 μmol/L, which is considered as secondary CA deficiency status. The serum CA levels did not differ among the etiologies of LC. Furthermore, the serum CA levels did not correlate with the severity of liver damage according to the Child-Pugh classification and the MELD scores. However, the serum CA levels were strongly associated with the markers (CRN and eGFR) of renal dysfunction.
Serum CRN is known as a useful biomarker of renal dysfunction because CRN is synthesized in the muscle tissue and excreted into the urine.23 The normal serum CRN range is usually 0.4-1.2mg/dL.22-24 Recently, the eGFR (which is derived from three factors: age, sex and serum CRN level) has been widely used as a tool for diagnosing CKD and also is an independent factor of mortality in patients with decompensated LC.22,25 In previous reports, the relationship between eGFR and serum CRN has shown good correlation in CKD patients, with 50 ml/min/1.73 mm eGFR having been almost equal to 2mg/dl serum CRN.22 However, in the present study, as shown in Figure 4, even the LC patients showed the normal serum CRN level were observed to experience a decrease in eGFR. These results suggest that the serum CA levels may be overestimated in LC patients in spite of their serum CRN levels being within the normal range. Because the serum CRN level is strongly associated with the volume of muscle, the serum CRN level will be underestimated, thus, showing PEM and/or sarcopenia. Recent reports have suggested that serum Cystatin C, which is not influenced by the muscle volume, has been recommended to calculate the eGFR even in LC patients. 26-28Therefore, our data suggest that the use of only serum CA levels cannot precisely estimate secondary CA deficiency status, even in LC patients with mild renal dysfunction.
Of special interest, the present study showed that the serum Ac-CA and Ac-CA to T-CA ratio had a significant positive correlation with serum FFA concentrations as well as our previous report.29 Serum FFA levels have been considered as an indicator of starvation status and have been higher in LC patients with PEM.30-32 Additionally, recent studies have indicated that nutritional management has improved MHE in LC patients and that starvation would be one factor contributing to the pathogenesis of MHE.33 The other hand, as FFA is known as a preferential source of energy in liver disease, infusion of lipid emulsion and CA administration have been shown the favorable effect of liver regeneration in rats.34-37 In the present study, we did not measure the daily intake volume of food, including meat or the body muscle volume using anthropometric measurement in LC patients. Moreover, serum CA level did not correlate with peripheral venous NH3 concentration. As arterial NH3 but not venous NH3 represents the precise concentration of NH3,38 further study is also needed to clarify the relationship among the serum CA level, nutritional status and ammonia metabolism in LC patients.
In conclusion, the present study suggests that the rate of secondary CA deficiency is low and that serum CA levels are within normal limits in the majority of LC patients, although serum CA status may be closely associated with renal dysfunction. Furthermore, the serum Ac-CA to T-CA ratio may be a useful marker for malnutrition in LC patients.
This study was supported in part by a Grant-in-Aid for Scientific Research in Japan (No. 25461009). We thank Miss Koko Moto date for her preparation of blood samples and clinical records of each patient.
Author declares that there is no conflict of interest.

©2016 Suzuki, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.