Journal of
eISSN: 2471-1381


Research Article Volume 2 Issue 1
1Jinan Baofa Cancer hospital, China
2TaiMei Baofa Cancer hospital, China
3Beijing Baofa Cancer Hospital, China
Correspondence: C Yu Baofa, Wangzhuang Industry Park, Changping, Postcode 102206, Beijing, China, Fax 8 6139 0640 1276
Received: November 27, 2015 | Published: February 26, 2016
Citation: Jian LI, Han W, Liu J, et al. Use of hapten combined double cytotoxic drugs for enhancing survival time in large and huge hepatocellular carcinoma with comprehensive umipic therapy. J Liver Res Disord Ther. 2016;2(1):16-27. DOI: 10.15406/jlrdt.2016.02.00019
Purpose: To evaluate overall survival benefit in treatment of large and huge hepatocellular carcinomas (HCC) by Ultra-Minimum Incision Personalized Intratumoral Chemoimmunotherapy (UMIPIC) compared to the Intra tumoral Chemotherapy (ITCT). Both methods include the use of two chemotherapy agents. The effect of hapten as an immune booster will also be analyzed.
Materials and methods: Patients with HCC were treated with UMIPIC or ITCT, both had same therapeutic procedure, UMIPIC had a proprietary regimen composed of components includes an oxidant, a two cytotoxic drugs and hapten; ITCT delivered the same drugs but excluded hapten. Survival data of 94 patients were evaluated (n=71 for UMIPIC, n=23 for ITCT). The tumor size was classified as large tumor (5 to 10cm) and huge tumor (>10cm), tumor sizes with liver function of Child A and B were analyzed to evaluate the correlation with overall survival time and survival rate.
Results: The study revealed a significant improvement for 10months of median overall survival in UMIPIC (Test) and 5 months in ITCT (control), respectively (P<0.05). The 6-month and 1-year survival rates of UMIPIC and ITCT were 80% vs. 32.3% and 46% vs. 22%, respectively with significantly improvement (P<0.05). The study revealed that UMIPIC has significant improvement in overall survival compared to that of ITCT for all size of HCC. There was no significant difference between patients with large and huge HCC; patient’s liver function in Child A had a better survival time compared to that of patient’s liver function in Child B in patients with UMIPIC therapy. CD3+, CD4+ and CD4+/CD8+ ratio increased following UMIPIC therapy indicating patient’s special immunity reaching a high level.
Conclusion: Hapten enhanced improvement in the survival time in patients with UMIPIC therapy compared to patients with ITCT therapy. Hapten also enhanced patient’s special immunity at a high level with CD4+ and CD4+/CD8+ ratio increasing. The study revealed hapten plays an important role in UMPIC therapy to improve patient’s survival time. Liver function was also increased as indicated by results of Child A and Child B.
Keywords: hapten, cd4+ and cd8+, hepatocellular carcinoma, intra tumoral chemo immunotherapy, ultra-minimum incision personalized intra tumoral chemo immunotherapy
HCC, hepatocellular carcinoma; TNM, tumor-node metastasis; UMPIC, ultra-minimum incision personalized intra tumoral chemo immunotherapy; AFP, serum alpha-fetoprotein; Alb, serum albumin; Tbil, total serum bilirubin; ALT, alanine amino transferase; EROTC, european organization for research and treatment of cancer; RECIST, response evaluation criteria in solid tumors; NCI, national cancer institute; CRF, case report forms; OS, overall survival; CR, complete response; PR, partial response; SD, stable disease; PD, progress disease; EM, extracellular matrix; PAMPs, pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; APCs, antigen-producing cells; DCs, dendritic cells
Hepatocellular carcinoma (HCC) is an aggressive cancer.1,2 An estimated 35,660 new cases and 24,550 cancer deaths will occur in the US in 2015.3 The current treatments for large or huge HCC, including TACE (TACE),4,5 adoptive immunotherapy,6 interferon therapy,7 percutaneous ethanol injection, radio-frequency ablation and a molecular target drug like Sorafenib8used either alone or in combination, showed benefits on survival rates but still showed limited outcomes.9 Current therapeutic approaches of TACE (oil-water drug emulsion) is often used in clinical practice of HCC therapy, however it belongs in local therapy with less side effects compared to systematic chemotherapy.
The concept of percutaneous intra tumoral drug delivery has been known for several decades as one of local therapies.10Some successful examples have clearly shown the clinical feasibility of such treatment options, with significant reduction in both toxicity and tumor growth. However, most research of intra-tumoral drug delivery with single drug had limited the clinical improvement on the survival time. The combination of different drugs in an aqueous solution for intra-tumoral therapy with or without hapten as an immunological booster has been utilized in application of intra tumoral drug delivery.10,11 Hapten like DNP was used in in-vitro melanoma autologous vaccine. Vaccine therapy in-vivo had shown significant improving of survival rate.11,12 Our published data suggests that UMIPIC offers an ideal percutaneous intra-tumoral approach for chemical de-bulking of advanced lung cancer and that hapten plays an important role in prolonging patients’ survival time.13
In the last decade we started the clinical research of therapeutic combination in an aqueous solution of single chemotherapeutic drugs and oxidant with hapten (as UMIPIC) or without hapten (as ITCT) in HCC patients using percutaneous intra-tumoral injection. The data showed that single and multiple cycles of UMIPIC therapy with single cytotoxic drug were analyzed and it revealed that multiple cycles have a better survival time than single treatment.14 The clinical research of combination with double chemotherapeutic drugs and oxidant with or without hapten as comparison study in HCC treatments is just finished. The primary objective of this cohort study was to assess the patient’s survival time of UMIPIC with double drugs vs. injection of ITCT. Another objective evaluated the role of hapten in UMIPIC therapy.
Patient selection and data collection
Patients were informed of the study procedure details and agreed to participate by signing informed consent and the ethics committee in author’s hospital (Ref. no.TMBFZLYY001) approved the study. Patient data was confirmed by imaging and pathological and cytological diagnosed cancer. Primary HCC patients with local advanced and/or metastatic cancer from November 2001 to September 2008 were analyzed. Randomized patients (2:1) received UMIPIC (2/3) and others (1/3) received ITCT both with double cytotoxic drugs. Data was collected with case report forms (CRFs) completed by hospital physicians. Collected data included clinical characteristics, follow-up time and survival data. For each patient the first follow-up visit was scheduled 6-8 weeks after treatment initiation and then on one-month intervals. Median follow-up time was 18 months. The records were updated during each follow-up visit. At the end of follow-up, survival data of 94 patients were evaluated (n=71 for UMIPIC, n=23 for ITCT). All tumors were large or huge in size, and all cases received multiple treatments. Most of them were staged as stage III (52.17% in ITCT VS. 47.8% in UMIPIC, P>0.05) and IV (47.88% in ITCT VS. 52.11% in UMIPIC, P>0.05) according to the tumor-node metastasis (TNM) classification, for 5 to 10cm diameter as large tumors and over 10 cm as huge tumors. The baseline characteristics of the patients were well balanced between the two groups with no significant difference (P>0.05) (Table 1).
ITCT |
UMIPIC |
|||||
|---|---|---|---|---|---|---|
N |
% |
N |
% |
|||
Enrolled patients |
23 |
100.00 |
71 |
100.00 |
||
Sex |
Male |
18 |
78.26 |
55 |
77.46 |
|
Female |
5 |
21.74 |
16 |
22.54 |
||
Hepatitis |
Hepatitis B |
11 |
47.83 |
34 |
47.89 |
|
Hepatitis A |
0 |
0.00 |
0 |
0.00 |
||
Hepatitis C |
0 |
0.00 |
0 |
0.00 |
||
None |
12 |
52.17 |
37 |
52.11 |
||
Alcohol |
11 |
47.83 |
39 |
54.93 |
||
Jaundice |
4 |
17.39 |
7 |
9.86 |
||
Albumin (g/L) mean (SD) |
37.9 |
38.31 |
||||
Total bilirubin (mmol/L) mean (SD) |
43.12 |
34.19 |
||||
Liver cirrhosis (%) |
12 |
52.17 |
38 |
53.52 |
||
AFP (μg/L) |
<20 |
13 |
56.52 |
43 |
60.56 |
|
20-400 |
7 |
30.43 |
11 |
15.49 |
||
>400 |
3 |
13.04 |
17 |
23.94 |
||
Stage of disease |
Stage I |
0 |
0.00 |
0 |
0.00 |
|
Stage II |
0 |
0.00 |
0 |
0.00 |
||
Stage III |
12 |
52.17 |
34 |
47.88 |
||
Stage IV |
11 |
47. 8 |
37 |
52.11 |
||
Tumor size |
<5cm |
0 |
0.00 |
0 |
0.00 |
|
5–10cm |
8 |
34.78 |
33 |
46.48 |
||
≥10cm |
15 |
65.22 |
38 |
53.52 |
||
Massive Hepatocellular carcinoma |
0 |
0.00 |
0 |
0.00 |
||
Diffuse Hepatocellular carcinoma |
0 |
0.00 |
0 |
0.00 |
||
Previous treatment |
Prior chemotherapy |
0 |
0.00 |
0 |
0.00 |
|
Prior adjuvant therapy |
8 |
34.78 |
25 |
35.21 |
||
Prior surgery |
0 |
0.00 |
0 |
0.00 |
||
Disease status |
Locally advanced |
11 |
47.83 |
37 |
51.38 |
|
Metastatic disease |
12 |
52.17 |
34 |
47.22 |
||
Tumor cases |
Invasion of the portal vein |
5 |
21.74 |
28 |
39.44 |
|
Invasion of liver capsule |
11 |
47.83 |
33 |
46.47 |
||
Ascites |
5 |
21.74 |
20 |
28.17 |
||
1 tumors |
12 |
52.17 |
29 |
40.85 |
||
≥2 tumors |
11 |
47.83 |
42 |
59.15 |
||
Child-Pugh |
A |
12 |
52.17 |
45 |
63.38 |
|
B |
10 |
43.48 |
23 |
32.39 |
||
C |
1 |
4.35 |
3 |
4.23 |
||
Table 1 Patient’s Characteristics.
UMIPIC and ITCT preparation
UMIPIC and ITCT have the same therapeutic procedure. The 25 Gauge spinal needles and the inflators (inflation device, 30atm/bar) were purchased from Merit-Medical, South Jordan, UT. UMIPIC and ITCT solutions were freshly prepared before each injection. UMIPIC contains clinically approved regimens (each regimen includes an old drug, an oxidant (H2O2), cytotoxic drugs with saturated concentration of adriamycin and bleomycin and a hapten) for percutaneous intra tumoral delivery. ITCT contains the same regimens, an oxidant with the cytotoxic drugs but without hapten.
Treatment delivery
All patients had either a pretreatment ultrasound or CT scan of the liver as a baseline. Routine examination of cardiopulmonary function was also done prior to treatment. The laboratory blood tests included hepatitis B and C virus antigen/antibodies, serum alpha-fetoprotein (AFP), serum albumin (Alb), total serum bilirubin (Tbil), and alanine aminotransferase (ALT) and Child A, B and C were used for classification. Patients with intestinal obstruction and any heavy infections were not allowed to receive this therapy until this symptom disappeared. Prior to UMIPIC or ITCT Therapy the patients were asked to fast without water intake for 14 hours pre-treatment in order to avoid side effects such as vomiting. In order to control pain that may occur during the treatment, 50 mg of morphine was injected intramuscularly at least 30 minutes prior to treatment for all patients. The skin was cleansed and local anesthesia performed in the area of injection.
After the spinal needle was inserted into the tumor under CT guidance, the core was taken out of the needle (which was connected to the inflator used as a high pressure syringe), then the injection was performed (Figure 1). UMIPIC or ITCT was delivered by a spinal needle inserted into the tumor and connected with the inflator for injection under pressure (at the level of atmospheric pressure or a little higher) to obtain fully forced distribution of clinically approved regimens in the tumor. Ultrasound or CT (Picker IQ, Phillips Healthcare, Bothell WA) guidance was used for scanning and monitoring of the density changes at a point or area of interest in the liver tumor. Special attention was paid to monitoring the CT value changes in the margins of surrounding tumor to ensure full distribution of drugs to the edge of the tumor (Figure 2). Since the combination solution is composed of water-soluble drugs with higher pressure (with inflator) for injection into the tumor mass and forced distribution in tumor, it is quite different from oil-water (drug) emulsion which is sticky and hard to distribute intratumorally in tumor’s mass. The combination of drugs in UMIPIC and ITCT could penetrate into the full matrix of the tumor, even into tumor cells, then therapeutic coagulation occurred with sustained release in the tumor for an extended time with the help of an oxidant.11,13 The average time of the procedure took approximately 30-45 minutes, however if the tumor was difficult to penetrate, a repeat CT was needed for monitoring and evaluating whether it needed more injection. The volume of the injection was calculated based on the diameter of the tumor (Dt) x 1.5 for over 5 cm of tumor, and maximal dose was 40 ml for huge tumor at each treatment time. Effective outcomes require multiple cycles of treatment even to over ten times of therapy. There are no other therapies following UMIPIC and ITCT therapies.
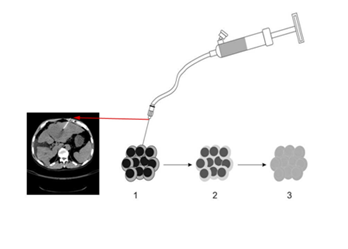
The procedure of the UMIPIC:
1) Guided by CT, the needle was inserted into tumor, connected to the inflator, and introduced intratumorally with the optimal route and angle;
2) The regimens was slowly delivered into the tumor;
3) With high pressure supplied by the inflator; the solution can penetrate into the extracellular matrix of tumor and facilitate forced diffusion.
The size of the tumor (tumor mass) is classified and re-examined by CT scanning for each week post-injection, the treatment is repeated at the time of reexamination.
Three treatments in total included the initial treatment as one cycle treatment of UMIPIC and ITCT (both as multiple treatment), one treatment per week. If the tumor size is not stabilized and smaller after six to eight weeks of initial treatment, additional cycle treatment were always added to promote improved efficacy. Some patients received three to five cycles of treatment. Some biopsis were taken during treatment for observation of pathological changes under electron microscopy. Patients are closely monitored for first day of each treatment to identify significant systemic or local adverse effects.
Assessment
The response to treatment was evaluated by solid tumor effect evaluation criterion of EROTC (European Organization for Research and Treatment of Cancer) and RECIST (Response Evaluation Criteria in Solid Tumors) made by NCI (National Cancer Institute) (US and Canada) in October 1999.15 All case report forms (CRF) were filled by treating physicians from hospitals.
Statistical analysis
Statistical analysis was performed. The primary objective was to evaluate the overall survival (OS), which was defined as the duration from the first treatment date to the death date and estimated according to the Kaplan-Meier analysis. Secondary end point time was response rate at four to six weeks post treatment, defined as the proportion of patients with a complete response (CR), partial response (PR), or stable disease (SD) and progress disease (PD) according to RECIST (version 1.0). Response rate and survival rate were analyzed and statistical differences between groups were based on the Chi-square test. The tumor size was classified as large tumor (5 to 10 cm) and huge tumor (>10cm), tumor sizes with liver function of Child A and B, local advantage and metastatic disease, tumor invasion of the portal vein or not, and tumor invasion of liver capsule (Table 1) All were analyzed to determine whether or not there was correlation with overall survival time. The statistical analysis was conducted with SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA); P value of <0.05 was considered to indicate statistical significance.
Efficacy evaluation
It was found that the response rates (CR+PR+SD/TOTAL) were 94.36% and 82.61% in UMIPIC and ITCT groups respectively and revealed no significant difference (P>0.05, Table 2). Stable disease (SD) was most common status of disease with UMIPIC and ITCT therapy. The slight size increase of the tumor mass observed in UMIPIC group was likely due to inflammatory response induced by therapeutic coagulations or interactions of malignant cells with the extremely high concentration cytotoxic drugs from the local administration.10,11 The inflammation in tumors was induced by intra-tumoral Chemoimmunotherapy in lung cancer patients. They were treated by same UMIPIC-Therapy with similarly effective rate and hepatocellular carcinoma with single drug in UMIPIC therapy,13,14 but the most tumors were found to be stable condition, some of tumors was CR and tumor necrosis.
Group |
N |
CR |
PR |
SD |
PD |
Effective Rate |
Benefit Rate |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
% |
χ2 |
P |
% |
χ2 |
P |
||||||
UMIPIC |
71 |
0 |
7 |
60 |
4 |
9.85 |
0.678 |
0.410 |
94.36 |
3.084 |
0.079 |
ITCT |
23 |
0 |
1 |
18 |
4 |
4.35 |
82.61 |
||||
Table 2 Comparison patient’s response rate and benefit rate.
CR: Complete Response; PR: Partial Response; SD: Stable Disease; PD: Progress Disease
The mean and median OS (censored observations) was 18 and 10 months in UMIPIC vs. 11 and 5 months in ITCT groups, respectively (Table 3). This represents a significant difference between the two groups (P<0.05), with curves (Kaplan-Meier) for both groups depicted in Figure 3-1, 3-2 & 3-3. The 6-month and 1-year survival rates of the UMIPIC and ITCT groups were 83.98% vs. 39.13% and 46.48% vs. 21.74% (Table 3, Figure 3-1, 3-2 & 3-3). This represents a significant improvement (P<0.001 to P<0.05) in UMIPIC and ITCT groups, with the only difference being the addition of hapten in UMIPIC compared to ITCT. This strongly indicated that the hapten enhanced immunological response in the body to guard against the potential tumor cells in the body therefore prolonging patient’s survival time.
Group |
N |
Mean |
Median |
Log-Rank |
6-Month Survival Rate |
1-Year Survival Rate |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
χ2 |
P |
% |
χ2 |
P |
% |
χ2 |
P |
||||
UMIPIC |
71 |
18.11 |
10 |
4.201 |
0.040 |
83.98 |
14.066 |
0.000 |
46.48 |
4.623 |
0.032 |
ITCT |
23 |
11.47 |
5 |
39.13 |
21.74 |
||||||
Table 3 Comparison patient’s survival time and rate in UMIPIC and ITCT.
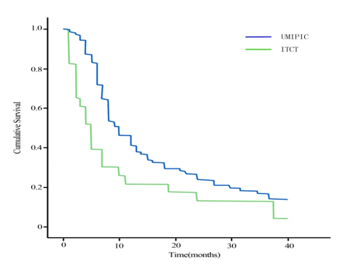
Overall survival (OC) curves in patients treated with UMIPIC vs. ITCT with double cytotoxic drugs. p= 0.000. It showed that the 6-months and 1-year survival rate in UMIPIC and ITCT were significantly difference and it revealed the hapten in therapy played a important role in prolong patient’s survival time.
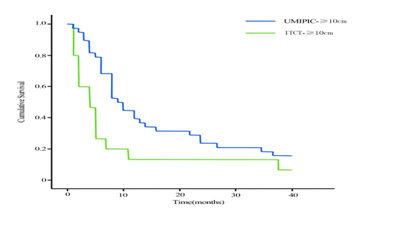
Overall survival (OS) curves in patients with huge tumor for more cycles of treatments between UMIPIC and ITCT with double drugs (P= 0.000). It showed that the 6-months and 1-year survival rate were significantly difference between UMIPIC and ITCT and it indicated the hapten in this therapy of UMIPIC played a important role in prolong patient’s survival time.
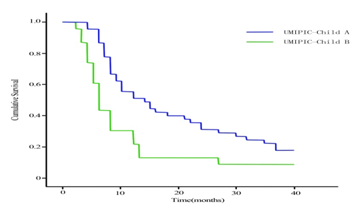
Overall survival (OS) curves in patients liver function in Child A and B in UMIPIC therapy (P= 0.000). It showed that the 6-months and 1-year survival rate in Child A were significantly improvement in Child B and it indicated the hapten in this therapy of UMIPIC for patient in Child A played an important role in prolong patient’s survival time.
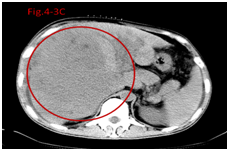
The tumor size was classified as small tumor (<5 cm, no small tumor in this study), large tumor (5 to 10 cm) and huge tumor (>10 cm), tumor sizes with liver function of Child A and B (Table 1) were analyzed to identify correlation with overall survival time (Table 4). It revealed that median survival time and rate of 6 months and 1 year in UMIPIC was not significantly different between large tumors and huge tumors, it also indicated that UMIPIC therapy could be effective against any size of tumors; it also revealed the median survival time and survival rate of 6 months and 1 year in UMIPIC has an significantly improvement compared to that of ITCT in huge tumors. The results indicated that hapten in UMIPIC can make a difference compared to ITCT in the treatment of huge tumors (Table 4). Several months after UMIPIC therapy, some patient’s tumors were smaller, in some tumors there were no changes, and it did not affect patient’s survival time (Figure 4-1,4-2,4-3).
Group |
N |
Mean |
Median |
Log-Rank |
6-Month Survival Rate |
1-Year Survival Rate |
|||||
χ2 |
P |
% |
χ2 |
P |
% |
χ2 |
P |
||||
|
|||||||||||
UMIPIC-5-10 |
33 |
17.52 |
10 |
0.029 |
0.864 |
87.88 |
1.003 |
0.317 |
48.48 |
0.100 |
0.752 |
UMIPIC-≥10 |
38 |
18.63 |
9 |
78.95 |
44.74 |
||||||
|
|||||||||||
ITCT-5-10 |
8 |
13.00 |
7 |
- |
- |
62.5 |
- |
- |
37.5 |
- |
- |
ITCT-≥10 |
15 |
10.67 |
4 |
26.67 |
13.33 |
||||||
|
|||||||||||
UMIPIC-5-10 |
33 |
17.52 |
10 |
- |
- |
87.88 |
- |
- |
48.48 |
- |
- |
ITCT-5-10 |
8 |
13.00 |
7 |
62.5 |
37.5 |
||||||
|
|||||||||||
UMIPIC≥10 |
38 |
18.63 |
9 |
4.170 |
0.041 |
78.95 |
12.782 |
0.000 |
44.74 |
6.132 |
0.023 |
ITCT ≥10 |
15 |
10.67 |
4 |
26.67 |
13.33 |
||||||
Table 4 Comparison tumor size (large and huge) inflection on survival time and rate.
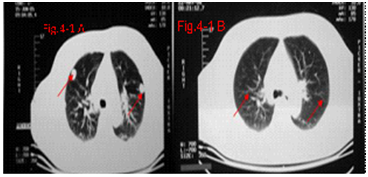
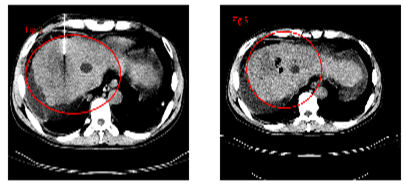
The “abscopal effect” of immunotherapy on the HCC patient with bilateral pulmonary metastases after 11 treatments of UMIPIC. The primary HCC tumor mass with diameter of 13.5 cm pre-treatment, primary HCC tumor mass with diameter of 5.2 cm post-treatment.
A: The bilateral pulmonary metastases pre-treat.
B: Regression of bilateral pulmonary metastases after 11 treatments of UMIPIC.
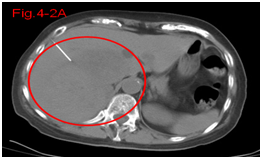
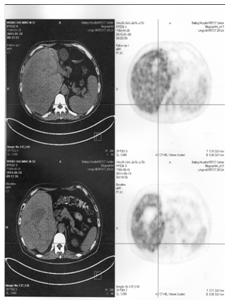
Figure 4.2A Before therapy CT showed huge big tumor.
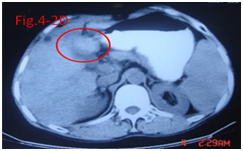

Figure 4.3A Before therapy CT showed huge big tumor and needle in tumor for injection.
The study also revealed that survival time including median time and survival rate of 6 months and 1 year survival rates in patient’s liver function with Child A in UMIPIC therapy was significant improvement over UMIPIC patient’s liver function with Child B and ITCT with Child A and B; there was no significant difference in Child A and B with ITCT therapy. There was no significant difference in patients with Child B for UMIPIC and ITCT therapy (Table 5).
Group |
N |
Mean |
Median |
Log-Rank |
6-Month Survival Rate |
1-Year Survival Rate |
|||||
χ2 |
P |
% |
χ2 |
P |
% |
χ2 |
P |
||||
|
|||||||||||
UMIPIC-A |
45 |
21.8 |
14 |
6.122 |
0.013 |
95.56 |
13.505 |
0.000 |
55.56 |
3.939 |
0.043 |
UMIPIC-B |
23 |
12.13 |
6 |
60.87 |
30.43 |
||||||
|
|||||||||||
ITCT-A |
12 |
12.41 |
4 |
0.060 |
0.807 |
50 |
0.903 |
0.342 |
33.33 |
1.691 |
0.193 |
ITCT-B |
10 |
11.20 |
5 |
30 |
10 |
||||||
|
|||||||||||
UMIPIC-A |
45 |
21.8 |
14 |
4.068 |
0.044 |
95.56 |
16.295 |
0.000 |
55.56 |
1.872 |
0.171 |
ITCT-A |
23 |
12.41 |
4 |
50 |
33.33 |
||||||
|
|||||||||||
UMIPIC-B |
23 |
12.13 |
6 |
0.503 |
0.478 |
60.87 |
2.659 |
0.103 |
30.43 |
1.585 |
0.208 |
ITCT-B |
10 |
11.20 |
5 |
30 |
10 |
||||||
Table 5 Comparison patient’s liver function inflection on survival time and rate.
In comparison with locally advanced and metastatic HCC (Table 6), the results revealed that locally advanced HCC has a better survival time (P<0.05) and survival rate of 6 months (P<0.01) and 1 year (P<0.01) than survival time and rate in patients with metastatic HCC in UMIPIC therapy groups, not in ITCT groups
Groups |
N |
Mean |
Median |
Log-Rank |
6-month survival rate |
1-year survival rate |
|||||
χ2 |
P |
% |
χ2 |
P |
% |
χ2 |
P |
||||
|
|||||||||||
Locally advanced |
24 |
23.63 |
18 |
4.094 |
0.043 |
100 |
7.374 |
0.007 |
70.83 |
8.645 |
0.003 |
Metastatic disease |
47 |
15.30 |
8 |
74.47 |
34.04 |
||||||
|
|||||||||||
Locally advanced |
11 |
12 |
5 |
0.229 |
0.632 |
45.45 |
0.354 |
0.552 |
27.27 |
0.379 |
0.538 |
Metastatic disease |
12 |
11 |
4 |
33.33 |
16.67 |
||||||
Table 6 Comparison survival time in locally advanced HCC and metastasis HCC in UMIPIC therapy.
Comparison with CD3+, CD4+ changes and CD4+/CD8+ ratio changes following the UMIPIC therapy (Table 7-1), showed increasing of CD3+, CD4+ and CD4+/CD8+ ratio; no changes in ITCT therapy (Table 7-2). It indicated that the patient’s immunological level go rose; this was associated with dendritic cell activity found (Figure 5-1–5-8).
|
n |
Average |
T value |
P Value |
|
||||
Before |
36 |
73.50 |
1.204 |
0.237 |
After |
36 |
75.13 |
||
|
||||
Before |
36 |
44.08 |
2.699 |
0.011 |
After |
36 |
48.06 |
||
|
||||
Before |
36 |
24.22 |
1.488 |
0.146 |
After |
36 |
23.44 |
||
|
||||
Before |
36 |
2.15 |
2.132 |
0.040 |
After |
36 |
2.38 |
||
Table 7.1 Comparison changes of CD3+, CD4+ and CD8+ following UMIPIC therapy.
|
n |
Average |
T value |
P Value |
|
||||
Before |
17 |
76.94 |
0.077 |
0.940 |
After |
17 |
77.11 |
||
|
||||
Before |
17 |
45.47 |
1.517 |
0.149 |
After |
17 |
49.57 |
||
|
||||
Before |
17 |
26.00 |
1.337 |
0.200 |
After |
17 |
24.82 |
||
|
||||
Before |
17 |
2.09 |
0.940 |
0.361 |
After |
17 |
2.66 |
||
Table 7.2 Comparison changes of CD3+, CD4+ and CD8+ following ITCT therapy.
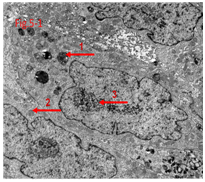
Figure 5.1 Loose reticular nucleus and increasing of lysosomes and autophagy corpuseles due to drug’s action with death tumor cells.
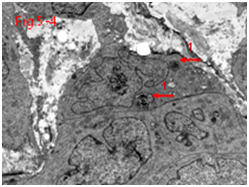
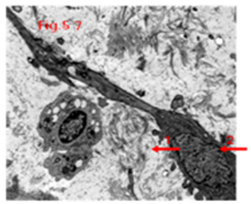
Other factors found not to impact survival time in UMIPIC therapy, such as hepatitis B, alcohol took, liver cirrhosis, APF, invasion of liver capsule, ascites and even tumor numbers (Table 8).
Group |
N |
Mean (Months) |
Median (Months) |
Log-Rank |
6-Month Survival Rate |
1-Year Survival Rate |
|||||
χ2 |
P |
% |
χ2 |
P |
% |
χ2 |
P |
||||
|
|||||||||||
Hepatitis B |
34 |
17.41 |
9 |
0.136 |
0.713 |
85.29 |
0.224 |
0.636 |
44.12 |
0.146 |
0.702 |
No Hepatitis B |
37 |
18.76 |
10 |
81.08 |
48.65 |
||||||
|
|||||||||||
Alcohol |
39 |
17.87 |
8 |
0.004 |
0.951 |
84.61 |
0.142 |
0.707 |
41.03 |
1.034 |
0.309 |
Non Alcohol |
32 |
18.40 |
12 |
81.25 |
53.13 |
||||||
|
|||||||||||
Liver cirrhosis |
38 |
18.40 |
9 |
0.010 |
0.922 |
84.21 |
0.072 |
0.788 |
47.37 |
0.004 |
0.952 |
Non-cirrhosis |
33 |
17.78 |
10 |
81.82 |
45.45 |
||||||
|
|||||||||||
AFP<20 |
43 |
16.61 |
10 |
0.666 |
0.415 |
81.40 |
0.225 |
0.235 |
46.51 |
0.000 |
0.995 |
AFP≥20 |
28 |
20.43 |
9 |
85.71 |
46.43 |
||||||
|
|||||||||||
Invasion liver capsule |
33 |
19.24 |
10 |
0.305 |
0.581 |
84.85 |
0.134 |
0.714 |
48.48 |
0.100 |
0.752 |
Non Invasion liver capsule |
38 |
17.13 |
8 |
81.58 |
44.73 |
||||||
|
|||||||||||
Ascites |
20 |
12.80 |
8 |
2.936 |
0.087 |
70.00 |
3.401 |
0.065 |
35.00 |
1.475 |
0.225 |
Non ascites |
51 |
20.20 |
12 |
88.24 |
50.98 |
||||||
|
|||||||||||
1 tumor |
29 |
19.17 |
12 |
0.102 |
0.749 |
93.10 |
3.494 |
0.062 |
55.17 |
1.489 |
0.422 |
≥2 tumors |
42 |
17.38 |
8 |
76.19 |
40.48 |
||||||
Table 8 Comparison other factors of inflection on survival time in UMIPIC therapy.
Common complications included temporary mild fever (not over 38℃) for a few hours, minor pain at injection area and minimal hemorrhage around the tumor and needle track after therapy were observed. No other significant systemic or local adverse effects were observed. Common chemotherapy side effects such as myeloid suppression, neutropenia, thrombocytopenia, GI toxicity, and apparent loss of hair and/or appetite were not seen.
Recently HCC is believed to be a potentially ideal tumor for targeting therapy by immune-based therapies,16therefore immunotherapy approaches may play a crucial role in addition of current treatment. To date the immunotherapeutic strategies for HCC have included the administration of immune stimulator cytokines,17 gene therapy with cytokines and co-stimulatory molecules,18 immunotherapy with dendritic cells loaded with specific tumor antigen19 and stimulation with immunogenic vaccines or antibodies.20 Effective combinations with immunotherapy and chemotherapy or TACE remains to be explored. Rescigno had showed that cytotoxic drugs are not always detrimental to immune system; they can actually enhance anti-tumor immune response by increasing tumor antigen presentation and depleting tumor-promoting regulatory T cells, as well as through other mechanisms.21Moreover, Chemoimmunotherapy has also demonstrated synergistic efficacy in the treatment of HCC,22 lymphoma23 and leukemia.24
TACE is now considered to be one standard of care worldwide for patients with primary HCC but limited on patients HCC with large and huge tumors and extra hepatic metastases.11,25 TACE can shut hepatic arterial flow toward tumor with help of oil-drug emulsion, while the drug leaves the oil to the tumor for killing the cancer cells, also the drug out of oil moves to the whole body too, that is why TACE produces side-effects which includes vomiting, loss hair and leukocytopenia in most HCC cases post treatment of TACE; there are few reports about this phenomenon. We developed a unique approach of UMIPIC that creates a drug depot in the tumor thorough a therapeutic coagulation with drug sustained release for prolonged time and avoiding circulation of drug to whole body. At this point it is better and different compared to TACE. UMIPIC integrates a therapeutic coagulation and chemotherapy drug simultaneously synergized with hapten, and produces tumor in-situ autologous vaccine likely function, another advantage over TACE which does not have this function. Clinically UMIPIC is performed much easier than TACE; it requires a biopsy and does not need a catheter and oil-drug emulsion.11,13 In general terms, UMIPIC can overcome the shortcomings of systematic chemotherapy or TACE; UMIPIC with single cytotoxic drug had successfully treated 214 advantaged of HCC and had extended patient survival time unprecedented.14
UMIPIC11,13,14in this clinical study is a patented therapeutic method for solid tumor, and was explored in this clinic with personalized dosage based on tumor-size while utilizing patient-specific in-vivo modified autologous tumor antigens of the patient as a self-vaccination to tumor-specific response.
Instantly during of UMIPIC treatment the oxidant can effectively change the extracellular matrix (EM) and alter morphological and biochemical components of the tumor cells such as collagen and other high-molecular-weight substances and results in a therapeutic coagulation in tumor mass. This leads to transformation into a soft, semisolid of tumor mass, stopping tumor metabolism and causing fibrosis (Figure 6). Coagulation could shut blood flow like TACE and entraps the injected drugs at higher concentrations within the coagulated tumors. The drug in water soluble base can penetrate into all matrix and tumor, even into tumor cells, which is not located in the blood vessels like TACE oil-water emulsion. This improves drug utilization by extending the duration of the drug in tumor and little drug systemic exposure through sustained drug release. Intratumoral therapy, as an obvious and attractive alternative to systemic treatment, has been approved for clinical use many years ago.26 In the clinical pharmacokinetic study, it revealed the drug retention at a high level in UMIPIC vs. in ITCT.14 Therefore, it is particularly important to note that the advantage of UMIPIC, including highly sustained and homogeneous forced cytotoxic drug diffusion in tumor can be obtained by injection with inflator at the level of atmospheric pressure or higher as it needed.
It is believed that the procedures of coagulation and drugs sustained in tumors play a powerful role in chemical de-bulking tumor main mass (more like Chemical surgery) (Figure 3) and provide an opportunity for the patient’s own immunotherapy to take place in the guardianship against micro tumors (no more than 108 tumor cells). The instant therapeutic coagulation of tumors can kill the Treg cells in a tumor mass following UMIPIC Therapy, and enhance up-regulation of T cells activities.
There is the "abscopal effect", that is the regression of distant tumor after localized treatment.27 In Dr. Yu’s earlier clinical study, hapten can induce an immunological abscopal-like antitumor response, as was documented in an HCC patient with bilateral pulmonary metastases.14 Charles Ludgate28 noted that pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) may have the desired abscopal effect on metastatic tumor sites by evoking an acute anticancer immune response using an "endogenous" vaccine approach. Similar results were mentioned in the clinical reports.29,30 The case with abscopal effect in (Figure 4), in fact, it may have inhibited any micro tumors in any place of the body delaying recurrent tumor development.
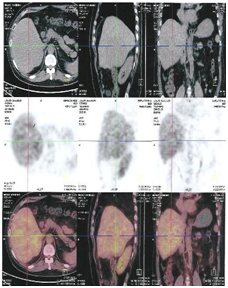
Figure 6A: Before UMPIC therapy, hepatocellular carcinoma had less radioisotope activity of F18 more activity in the tumor mass with PEC/CT, it means that tumor with less activity and less inflammation.
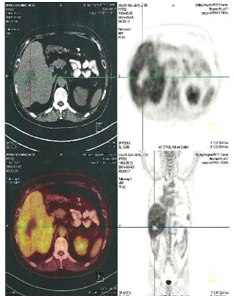
In the primary tumor’s coagulation and metabolism, multiple autologous tumor-associated antigens are slowly released from those dead tumor cells locally, which continue to trigger a mild immune response as self-vaccination. That is to say the quantity of the autologous tumor-associated antigens is just enough to reach the threshold required for stimulating immune response. Our earlier studies showed that there is a significant boost to systemic immunity after UMIPIC with single drug, especially for the number of CD4+ and CD8+ T cells.14 Cell death can be a priming event for T cell response and induce potent immunity.31,32 In the presence of an immunological modulator (i.e. small-molecule hapten inlaying the denatured tumor), the lysed tumor cells or antigens in the depot of coagulation were modified with hapten and generated stronger tumor-associated antigens referred to as an autologous tumor in-situ vaccine (making the tumor itself more immunogenic). Accordingly, the systemic immunity against patient-specific tumor-associated antigens was boosted by significantly increasing active antigen-producing cells (APCs) (including dendritic cells (DCs) and macrophage), which is recognized by T cells and NK cells. This fights active (or pathogenic) tumor cells in and around the primary tumor, as active tumor cells are not yet killed by initial coagulation, and also may kill active tumor cells in any part of the patient’s body.
Inflammatory response may involve anti-tumor immunity, the therapeutic coagulation mass may play as an inflammatory tissue with cytokine and chemokines.14 This clinical study revealed that UMIPIC with double drugs could induce more inflammatory response in local tumors, with no significant different response effect of UMIPIC and ITCT groups at the time of re-examination for evaluation of response rate. We believe that UMIPIC-induced inflammatory response with hapten may play an important role in the tumor’s autologous in-situ vaccine-like function, for patient with large and huge tumors. This immunological power may be weak to reach significant clinical benefit, so that multiple and high dosage of treatments are strongly needed to de-bulk tumor mass to the level of tumor load for immunotherapy to take place (Figure 5) (Figure 6). In our study, multiple and high double drug dosages of treatments have demonstrated clinical efficacy and safety with compelling clinical evidence (Table 3). The results revealed that median OS was ten months, and six months and one year survival rates were 80% and 46% respectively. Single drug in UMIPIC reported median OS was seven months, six months and one year survival rates were 58% and 30% respectively.14 Use of double drugs in UMIPIC gives an additional three months of life, a big improvement for prolonging of survival time. This may attribute to long-term immunological memory induced by constitutively-released antigens leading to a more effective anti-tumor response. An increasing of CD3+ CD4+ changes and CD4+/CD8+ ratio indicated the patient’s special immunity at a high level following UMIPIC therapy (Table 7). Furthermore, as the marker of the activated T cells, elevation of the co-stimulator may associate with clinical benefits and overall survival.33
With the concept of immunological therapy (hapten) and chemical surgery into UMIPIC as one comprehensive therapy, this therapy can have an effect on huge size of HCC. In this study of large and huge tumors, double drugs in UMIPIC showed a significant improvement in survival time compared to that of ITCT. UMIPIC also provided a better chance for immunological power to take place with the help of hapten after chemical surgery of huge mass of tumor, correlated with patient’s special immunity at a high level. Patient’s liver function in Child A has a better survival improvement benefit than Child B in UMIPIC therapy, and it also suggested that improvement of liver function is an important role in improvement of patient’s survival time. Compared with traditional chemotherapy, the side effects of UMIPIC involves minimal fever, reasonable local pain and the clinical outcome is an improved quality of life. Locally advanced HCC has a better survival time than that of metastatic HCC whether or not size of tumor changes with treatment.
In conclusion, UMIPIC provides a new way to decrease the cardinal mass while boosting the patient’s own immunological power to fight against micro tumor cells in a specific and innovative manner. This is one of advantages over TACE; another advantage is that UMIPIC is not limited to tumor’s sizes. The number and locations of tumors in the liver may not be suitable for TACE but can be addressed with UMIPIC. It could be concluded that UMIPIC with double drugs can surpass TACE in treatment for large and huge HCC, especially survival times in HCC. Some may be suitable for TACE therapy, however UMIPIC it can take the place of TACE in those who are is not suitable for TACE therapy or in failure of TACE. More effective controls of huge of HCC are strongly needed. We hope to continue to investigate UMIPIC Therapy with new double cytotoxic drugs with hapten or more haptens for better effectiveness; it may produce stronger and varied types of immunological responses to eradicate the residual ones of tumor and give more effectiveness to huge HCC treatment.
This work was supported by Baofa Cancer Hospital Groups, China. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author declares that there is no conflict of interest.

©2016 Jian, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.